Long-term outcome for achalasia in patients who
underwent laparoscopic Heller myotomy with Dor fundoplication
Mohammad
Taghi Ashoobi 1, Mohammad Sadegh Esmaeili Delshad 1,
Afshin Shafaghi 1, Manouchehr Aghajanzadeh 1*
1 Inflammatory
Lung Diseases Research Center, Department of Internal Medicine, School of Medicine,
Razi Hospital, Guilan University of Medical Sciences, Rasht, Iran
*Corresponding Author: Manouchehr Aghajanzadeh
* Email: manouchehr.aghajanzadeh.md@gmail.com
Abstract
Introduction: Achalasia is a rare esophageal motility disorder that can require
surgical intervention in some cases. This retrospective cross-sectional study
aims to evaluate the clinical symptoms of patients with advanced achalasia who
underwent laparoscopic Heller myotomy (LHM) and Dor fundoplication.
Materials and Methods: The study included 86 patients (38 men, 48 women) diagnosed with
achalasia between 2010 and 2020, of which 20 patients with advanced achalasia
underwent LHM and Dor fundoplication. The median follow-up time was 48 months.
Results: The study found that LHM and Dor fundoplication surgery improved
dysphagia in 12 patients, with four patients showing improvement in solid food
dysphagia and two patients showing improvement in semi-solid dysphagia.
Nocturnal cough and slow emptying sensation also improved in 16 cases.
Additionally, barium stasis decreased significantly in 14 patients. However,
two patients who underwent esophagectomy had hospital mortality.
Conclusion: This study highlights the effectiveness of LHM and Dor fundoplication
in reducing dysphagia, nocturnal coughing, regurgitation, and other obstructive
symptoms in patients with advanced achalasia. However, the study also
underscores the potential risks associated with esophagectomy, suggesting that
surgical treatment for achalasia should be carefully considered on a
case-by-case basis.
Keywords: Achalasia, Dysphagia, Heller myotomy, Fundoplication, Gastroesophageal
reflux
Introduction
Achalasia is an uncommon but quintessential esophageal motility
disorder that occurs equally in men and women (1). Achalasia
characterized by reduced relaxation of the lower esophageal sphincter (LES) and
absence of esophageal peristalsis resulted in impaired bolus transit,
demonstrated with symptoms including dysphagia, retrosternal pain,
regurgitation, and weight loss (2).
The disease’s pathogenesis is unclear and often misdiagnosed (3). Still, it is
suggested to happen because of a virus-related inflammatory neurodegenerative
process triggered by an autoimmune and chronic inflammatory process, especially
in patients with genetic susceptibility (4). However, at
the time of diagnosis, the number of decreased neurons led to significant
dysfunction and symptoms. Therefore, the first step of diagnosis is performing
endoscopy or radiology, but the gold standard diagnostic method for achalasia
is high-resolution manometry (HRM) (5).
According to Chicago classification, achalasia is classified into
three subtypes, type I (classic achalasia) refers to the one without any
significant pressurization in esophageal, type II is achalasia with
compression, which there is no peristalsis and contractile activity, and
pan-esophageal pressurization >30 mmHg, and type III is spastic achalasia
with rapidly propagated pressurization attributable to an abnormal lumen
obliterating contraction (6).
As achalasia progresses, dilation of the esophagus worsens and can
resemble a sigmoidal shape. In the end stage of achalasia, patients present
dilation of the esophagus with a sigmoid shape (7).
Unfortunately, there is no promising treatment for achalasia due to its unknown
pathogenesis, and standard treatment options include pharmacological therapy
(nitrates and calcium channel blockers), pneumatic dilation, endoscopic myotomy
(2,3), Botulinum
toxin (Botox) (8), surgical
myotomy, and esophagectomy (2,3).
Surgical treatment of achalasia has evolved dramatically over the
past 13 years. Since the first report of laparoscopic Heller myotomy by
Cuschieri and thoracoscopic Heller myotomy by Pellegrini, minimally invasive
surgery has become the gold standard for treating achalasia (9). More
recently, the laparoscopic management of esophageal achalasia has achieved
widespread acceptance and is now the first line of therapy for patients with
achalasia. The satisfactory short-term results of this procedure are well
documented in several large series.
Esophagectomy is more aggressive and associated with more
significant morbidity/mortality than laparoscopic Heller myotomy (LHM) and Dor
fundoplication (10). In this
regard, we study the post-surgical side effects and clinical symptoms of
patients in two groups who underwent LHD and Dor fundoplication in patients with achalasia.
Materials and Methods
This retrospective cross-sectional study was
conducted on 86 patients with achalasia in Razi Hospital, Rasht, Iran, from October 2010 to September
2020. The achalasia was confirmed by clinical findings (endoscopy, radiology, and HRM
results). In addition, all demographical data and clinical characteristics of
patients were recorded from the patient’s archive in the hospital.
The surgery approach was LHM (8 cm over the esophagus and 3 cm over
the stomach) and Dor fundoplication (Figure 1). All remnant food was aspirated
to prevent pulmonary aspiration after induction of general anesthesia with a
tracheal tub. Before the surgery, 16 of the patients had undergone previous
dilatations or Botox injections. Longitudinal and circular muscle of the
esophagus was cut on the last 8 cm of the esophagus and extended three cm on
the gastric wall musculature. Dor fundoplication was performed in all patients.
In our study, the perforation and complete myotomy were checked after
completion of cardiomyotomy with an ambo-bag, and via a tube in the esophagus
air inflate. Postoperative assessments include clinical, radiologic,
manometric, and endoscopic evaluation was performed.
A flap of the stomach for coverage was fixed to prevent diverticula
formation in the motorized site. Pre and post-operative assessment included
symptoms, esophageal emptying observation with barium esophagogram, HMR, and
endoscopic evaluation in all patients. The barium esophagogram was obtained
under fluoroscopic control.
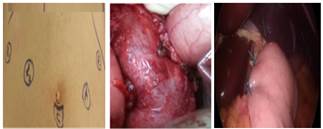
Figure 1. Laparoscopic
Heller myotomy in patients with achalasia.
The surgical technique for laparoscopic Heller myotomy was after
the pharyngoesophageal ligament that divided the fat pad excised and exposing
the anterior gastroesophageal junction; the myotomy was performed by incising
the distal 4 to 6 cm of esophageal musculature. Then, the myotomy was extended
2 to 3cm onto the gastric cardia using cautery scissors with an
intraesophageally tube; when the EJ junction closed, the esophagus was
inflated, mucosal perforations were detected, and the myotomy added a Dor
anterior hemifundoplication. Routinely, a contrast swallow was performed on the
second day of postoperative in all patients to rule out an occult leakage. For
patients with no leak, a clear liquid diet was started on the second
postoperative day, and all patients were discharged four days postoperatively.
The results were reported in number and percentage.
Results
Among a total number of 86 patients (38 males, 48 females) with a
median age of 46 years old, patients had advanced achalasia including lumen
dilatation of esophagus between 6 to 12 cm, moderate to severe intra luminal
stasis of barium, severe tortoise, recurrent pulmonary aspiration, and
recurrent pulmonary infection; and underwent laparotomy for achalasia. These
patients failed in pneumatic dilatation and Botox treatment. Dysphagia
presented in all patients, and 20 patients experienced an average weight loss
of 10 kg before surgery. Pre-surgical clinical characteristics of patients are
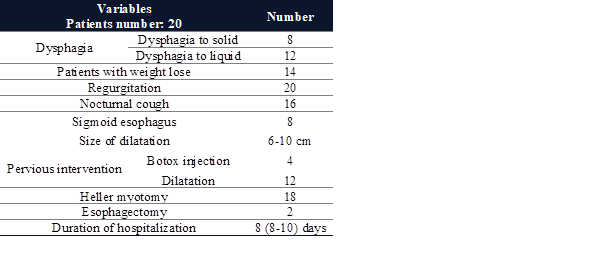
demonstrated in Table 1.
Table 1. Pre-surgical
clinical characteristics of patients with achalasia.
Two patients expired, one during operation and another one in five
days after surgery due to pneumonia and reparatory failure. Two patients
required reoperation for bleeding and gastric herniation. Six patients
experienced minor postoperative morbidity, including atelectasis (3n), atrial
tachyarrhythmia (4n), and wound infection (3n). The median follow-up days
were 30 months (10–48 months).
According to our results, stasis was reported in all patients
before the operation. The LES gradient decreased from 32 to 12 mmHg. Endoscopy
and biopsy findings demonstrated grade I esophagitis in four patients. Radiological findings represented that barium
stasis decreased from 92% to 22%. The post-surgery diameter of the esophagus
lumen was 8 cm (8–12 cm), which fell to 6 cm (6-10 cm). Body weight increased
after the myotomy [preoperative: 58 kg (38–83 kg), postoperative: 66 kg (48–86
kg)]. No diverticular formation was observed in the motorized zone. Short and
long-term functions and symptom improvement in patients with achalasia are
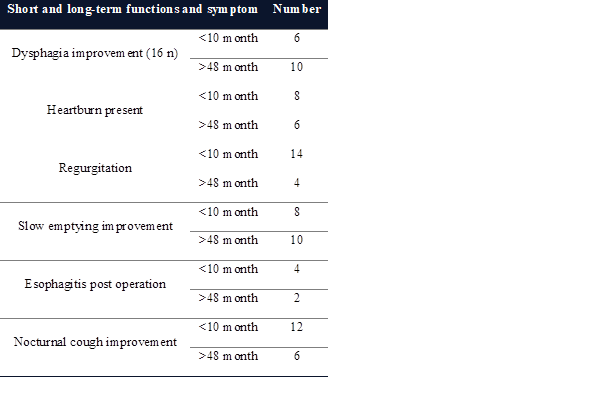
illustrated in Table 2.
Table 2. Short and long-term functions and symptom improvement in patients
with achalasia.
Discussion
Achalasia is a rare esophageal motility disorder for which there is
no known etiology, making treatment options challenging. The main goal of
treatment is to reduce LES pressure, improve dysphagia and regurgitation,
enhance esophageal emptying, and prevent the development of megaesophagus.
Surgical management of advanced achalasia is challenging, and esophagectomy is
associated with a high incidence of postoperative respiratory complications
such as pneumonia. Our results aillustrated that LHM is an effective treatment
with a higher patient survival rate and fewer complications.
Previous studies have reported that higher LES resting pressure is
associated with better relief of dysphagia after myotomy. The LHM–Dor procedure
provides satisfactory long-term results with low morbidity (11,12). Esophagectomy was associated with
a high incidence of postoperative respiratory complications, including
pneumonia, while LHM is more effective with a higher patient survival rate (13,14). Arain et al. reported that higher
LES resting pressure is associated with better relief of dysphagia after
myotomy (15). A study demonstrated that
extending myotomy three cm over the stomach reduces the postoperative pressure
on the LES with no significant difference in reflux when added an anti-reflux
procedure (16). Also, Liu et al. reported that
esophageal myotomy for achalasia could reduce the resting pressures of the
esophageal body and LES and improve esophageal transit and dysphagia (17).
Dor fundoplication added to myotomy reduces the risk of pathologic
gastroesophageal reflux, and our study showed that it could be performed in all
patients with a low incidence of reflux. Studies have reported favorable
responses in more patients even after a long term of follow-up. LHM and Dor
fundoplication balance emptying and reflux and could be the selected surgical
treatment for patients with achalasia (12,18). In an investigation on a series of
73 patients treated with LHM, favorable responses were reported in more than
half of the patients, even after over six years of follow-up (19). Siow et al. demonstrated in their
study that LHM and anterior Dor fundoplication are both safe and effective as a
definitive treatment for treating achalasia cardia with high patient
satisfaction with minimum complications (20). A study by Finley et al. reported
that 24 patients who underwent LHM without fundoplication had more significant
improvement in esophageal clearance time (21).
Rice et al. represented that the addition of Dor
fundoplication decreases the capability of LHM without impairing emptying and
reduces reflux. LHM and Dor fundoplication balance emptying and reflux, which
could be the selected surgical treatment for patients with achalasia (22). Kummerow et al. illustrated no
statistical difference between patient-reported dysphagia or reflux scores in
those who underwent an LHM with and without Dor fundoplication (23). In this present study, reflux was
reported in nine patients with Do fundoplication. Also, end-stage achalasia
treated by LHM with Dor fundoplication showed reduced LES gradient, decreased
obstructive symptoms, and improved esophageal emptying.
Performing LHM is an effective treatment with good dysphagia relief
and a low incidence of esophageal mucosal perforation (24). Abovementioned studies reported
that LHM is an effective treatment with good dysphagia relief and a low
incidence of esophageal mucosal perforation. While manometry is sometimes essential
for good surgical outcomes, long-term follow-up on dysphagia relief and patient
satisfaction is necessary to ensure the effectiveness of therapy. Overall, our
study showed that LHM and Dor fundoplication are safe and effective treatments
for advanced achalasia, providing significant improvement in obstructive
symptoms, decreased LES gradient, and improved esophageal emptying.
Limitations
The limitation of this study was the limited access to the history
of patients’ underlying disease and incomplete data on individuals’ diets and
lifestyles.
Conclusions
LHM provided satisfactory symptom
improvement in patients with advanced achalasia with promising outcomes. Also,
further investigations are required to demonstrate the most effective methods
in patients with severe achalasia.
Author contribution
MTA and MA wrote the main manuscript text and designed the
study. MSES and ASh. cooperated in data collecting and analysis.
All authors reviewed the manuscript.
Conflict of interest
The authors reported no potential conflict of interest.
Ethics approval
Relevant ethical guidelines and regulations were performed for all
experiments. This study was done
according to the Declaration of Helsinki ethical standards and consent and
agreement was obtained from all the patients and was confirmed and approved in
the surgery department.
Acknowledgments
We want to thank all hospital staff and specialists for their assistance with
conforming and recording cases.
References
1. Vaezi MF, Pandolfino
JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am
J Gastroenterol. 2013 Aug;108(8):1238–49; quiz 1250.
2. Torresan F, Ioannou A,
Azzaroli F, Bazzoli F. Treatment of achalasia in the era of high-resolution
manometry. Ann Gastroenterol Q Publ Hell Soc Gastroenterol. 2015;28(3):301.
3. Vaezi MF, Felix VN,
Penagini R, Mauro A, de Moura EGH, Pu LZCT, et al. Achalasia: from diagnosis to
management. Ann N Y Acad Sci. 2016 Oct;1381(1):34–44.
4. Boeckxstaens GE.
Achalasia: virus-induced euthanasia of neurons? Vol. 103, Official journal of
the American College of Gastroenterology| ACG. LWW; 2008. p. 1610–2.
5. Pandolfino JE, Fox MR,
Bredenoord AJ, Kahrilas PJ. High‐resolution manometry in clinical practice:
utilizing pressure topography to classify oesophageal motility abnormalities.
Neurogastroenterol Motil. 2009;21(8):796–806.
6. Rohof WOA, Bredenoord
AJ. Chicago Classification of Esophageal Motility Disorders: Lessons Learned.
Curr Gastroenterol Rep. 2017 Aug;19(8):37.
7. Hammad A, Lu VF, Dahiya
DS, Kichloo A, Tuma F. Treatment challenges of sigmoid-shaped esophagus and
severe achalasia. Ann Med Surg. 2021 Jan;61:30–4.
8. Ramzan Z, Nassri AB.
The role of Botulinum toxin injection in the management of achalasia. Curr Opin
Gastroenterol. 2013;29(4):468–73.
9. Torquati A, Richards
WO, Holzman MD, Sharp KW. Laparoscopic myotomy for achalasia: predictors of
successful outcome after 200 cases. Ann
Surg. 2006 May;243(5):583–7.
10. Yano F, Omura N, Tsuboi
K, Hoshino M, Yamamoto S, Akimoto S, et al. Learning curve for laparoscopic
Heller myotomy and Dor fundoplication for
achalasia. PLoS One. 2017;12(7):e0180515.
11. Andrási L, Paszt A,
Simonka Z, Ábrahám S, Erdős M, Rosztóczy A, et al. Surgical Treatment of
Esophageal Achalasia in the Era of Minimally Invasive Surgery. JSLS
J Soc Laparoendosc Surg. 2021;25(1).
12. Kashiwagi H, Omura N.
Surgical treatment for achalasia: when should it be performed, and for
which patients? Gen Thorac Cardiovasc
Surg. 2011 Jun;59(6):389–98.
13. Kahrilas PJ, Pandolfino
JE. Treatments for achalasia in 2017: how to choose among them. Curr Opin
Gastroenterol. 2017;33(4):270.
14. Schlottmann F, Patti MG.
Prevention of postoperative pulmonary complications after esophageal
cancer surgery. Vol. 11, Journal of
thoracic disease. China; 2019. p. S1143–4.
15. Arain MA, Peters JH,
Tamhankar AP, Portale G, Almogy G, DeMeester SR, et al. Preoperative lower
esophageal sphincter pressure affects outcome of laparoscopic esophageal
myotomy for achalasia. J Gastrointest Surg. 2004;8(3):328–34.
16. Oelschlager BK, Chang L,
Pellegrini CA. Improved outcome after extended gastric myotomy for achalasia.
Arch Surg. 2003 May;138(5):490–7.
17. Liu J-F, Zhang J, Tian
Z-Q, Wang Q-Z, Li B-Q, Wang F-S, et al. Long-term outcome of esophageal myotomy
for achalasia. World J Gastroenterol. 2004 Jan;10(2):287–91.
18. Vaezi MF, Pandolfino JE,
Yadlapati RH, Greer KB, Kavitt RT. ACG Clinical Guidelines: Diagnosis and
Management of Achalasia. Am J Gastroenterol. 2020 Sep;115(9):1393–411.
19. Ates F, Vaezi MF. The
Pathogenesis [1] F. Ates, M.F. Vaezi, The Pathogenesis and Management of
Achalasia: Current Status and Future Directions., Gut Liver. 9 (2015) 449–463.
https://doi.org/10.5009/gnl14446.and Management of Achalasia: Current Status
and Future Directions. Gut Liver. 2015 Jul;9(4):449–63.
20. Siow SL, Mahendran HA,
Najmi WD, Lim SY, Hashimah AR, Voon K, et al. Laparoscopic Heller myotomy and
anterior Dor fundoplication for achalasia cardia in Malaysia: Clinical outcomes
and satisfaction from four tertiary centers. Asian J Surg [Internet].
2021;44(1):158–63. Available from: https://doi.org/10.1016/j.asjsur.2020.04.007
21. Finley RJ, Clifton JC,
Stewart KC, Graham AJ, Worsley DF. Laparoscopic Heller myotomy improves
esophageal emptying and the symptoms of achalasia. Arch Surg.
2001;136(8):892–6.
22. Rice TW, McKelvey AA,
Richter JE, Baker ME, Vaezi MF, Feng J, et al. A physiologic clinical study of
achalasia: should Dor fundoplication be added to Heller myotomy? J Thorac
Cardiovasc Surg. 2005;130(6):1593–600.
23. Kummerow Broman K,
Phillips SE, Faqih A, Kaiser J, Pierce RA, Poulose BK, et al. Heller