Clinicopathological
response assessment to neoadjuvant chemotherapy in locally advanced breast
cancer- A rural population-based case series
Kailash
Kumar Thakuria 1, Sushmita Ray 2*, Rahul Sarma 2
1 Kokrajhar Medical College and hospital,
Assam, India
2 Fakhruddin Ali Ahmed Medical College and
hospital, Barpeta, Assam, India
Corresponding Author: Sushmita Ray
* Email: 1994shri@gmail.com
Abstract
Introduction: Breast cancer is the commonest malignancy among women worldwide.
Despite a multidisciplinary approach, locally advanced breast cancer remains a
clinical challenge as most of the patients have a high rate of locoregional
spread and develop distant metastases. Neoadjuvant chemotherapy not only paves
the way for a more conservative surgical option but also decreases the
incidence of positive nodes. This study was undertaken, to assess the
effectiveness of neo-adjuvant chemotherapy and its impact on clinical and
pathological response in locally advanced breast cancer. It also, compare
patient characteristics, histological type, and hormonal receptor status with
response to neo-adjuvant chemotherapy.
Materials and Methods: This is a
prospective observational study over a one-year period on 30 locally advanced
breast cancer patients from rural background who received neoadjuvant
chemotherapy. All patients received a standard neoadjuvant treatment regimen
and were evaluated clinically, radiologically, and pathologically pre- and
post-chemotherapy. The clinical response was assessed by RECIST criteria, the
pathological response was graded according to Chevalier classification, and the
overall impact was assessed by AJCC response criteria.
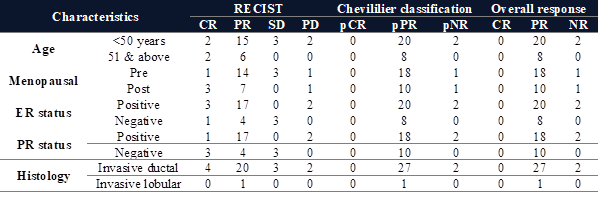
Results: Most of the patients (46.7%) were in the age group of 35-48 years. The
premenopausal and postmenopausal groups were 63% and 37%, respectively. In the
present study, tumours expressing oestrogen,
progesterone, and HER 2 were 73%, 66%, and 27%, respectively. Patients showing
clinically complete responses post-neoadjuvant chemotherapy were 4, partial
responses were 21, stable disease was 3, and progressive disease was 2. A
pathological partial response was achieved in 93% of patients.
Conclusion: Neoadjuvant chemotherapy in locally advanced breast cancer not only
downstages the disease but increases the scope of operability and thus makes it
possible to resect the disease with a tumour-free margin in most cases.
Keywords: Locally advanced breast cancer (LABC), Neoadjuvant chemotherapy (NACT)
Introduction
Breast cancer is among the most common cancers in
women worldwide. In Globocan India 2020, breast
cancer accounted for 13.5% of all new cancer cases in both sexes and 10.6% of
all deaths, with a cumulative new case risk of 2.81 (1). The National Comprehensive Cancer Network describes
locally advanced breast cancer (LABC) as American Joint Committee on Cancer
(AJCC) (7th edition) stage III breast cancer in the absence of distant
metastasis as tumours more than 5 cm in size with regional lymphadenopathy
(N1–3) or of any size with direct extension to the chest wall (T4a) or skin
(T4b) regardless of regional lymphadenopathy or presence of regional
lymphadenopathy (clinically fixed or matted axillary lymph nodes, or any of
ipsilateral infraclavicular, supraclavicular, or internal mammary
lymphadenopathy) regardless of tumour stage. In the AJCC 7th edition,
ipsilateral supraclavicular lymphadenopathy was reclassified as regional
lymphadenopathy (N3), which was considered in the AJCC 6th edition as distant
metastasis (2).
Despite widespread awareness of the benefits of
screening and early detection, 10% to 20% of breast cancer patients are
diagnosed with locally advanced disease in industrialized nations, compared to
up to 50% of incidence cases in developing nations (3). The present study is based on a rural-based
population who were diagnosed as LABC on initial presentation and is amenable
to less attrition in our health care setup.
Studies show that with only treatment with radical
mastectomy or roentgen, the five-year survival rate was 30% (4,5). Neoadjuvant chemotherapy became a part of standard
treatment for LABC after the National Surgical Adjuvant Breast and Bowel
Project B-18 trial, which found that it not only reduced tumour size but
decreased the incidence of positive nodes and also showed benefit in assessing
tumour response to it in vivo (6).
Histopathological response to primary chemotherapy was
found to be the single most important prognostic factor for both disease-free
and overall survival rates. Moreover, the extent of the remaining tumour
determines the rate of local recurrence and dictates the necessity for
additional loco-regional therapy (7).
Several studies have shown that oestrogen
receptor-negative patients achieved a higher pathological complete response
than positive patients (8). High tumour grade and tumour size were clinical
determinants of pathological complete response (9).
The present study was undertaken to assess the effectiveness
of neo-adjuvant chemotherapy and its impact on clinical and pathological
response in locally advanced breast cancer and to compare patient
characteristics, histological type, and hormonal receptor status with response
to neo-adjuvant chemotherapy (NACT).
Materials and Methods
After
getting the ethical approval of the project, consent and agreement were
obtained from all the patients. In this way, the study was conducted
prospectively on 30 rural background-based patients attending the surgery outpatient
department of Fakhruddin Ali Ahmed Medical Hospital, Barpeta,
Assam, India, from September 2021 to August 2022.
All
patients above 18 years of age diagnosed with locally advanced breast cancer
who were willing to undergo follow-up were included in the study. Operable
Locally advanced breast cancer (T3N1M0), history of prior radiotherapy to the
breast, the presence of distant metastases, and patients in whom neoadjuvant
chemotherapy is contraindicated were excluded from the study.
Patients
presenting with breast lumps were evaluated clinically, radiologically, and
pathologically by tru-cut biopsy to confirm the
primary tumour as locally advanced breast cancer. The
patient baseline and tumour characteristics,
including age, menstrual status, tumour size, nodal
status, tumour grade, hormonal receptor HER2 status,
and histological type, were noted, and metastatic workup was done.
Patients
were treated with a standard neoadjuvant chemotherapy regimen of
5-fluorouracil, epirubicin, and cyclophosphamide
(FEC). In triple negative cases, dose-dense AC followed by taxane
drugs (Paclitaxel or docetaxel) was given. In HER2-positive cases, AC followed
by taxane with trastuzumab and or pertuzumab
was given.
After
the completion of 3-4 cycles or additional neoadjuvant chemotherapy as needed,
the tumour was assessed clinically, and
radiologically for tumour size, nodal status, and
clinical stage, after which all the patients underwent modified radical
mastectomy with en bloc axillary dissection, and a
specimen was sent for gross and histopathological examination. Clinical response was assessed by RECIST criteria (response
evaluation criteria in solid tumours) (Table 1.)
Table 1. RECIST criteria for clinical
response evaluation.

Histopathological response is graded according to Chevalier
classification (9).
Table 2. Chevalier classification for grading
histopathological response.
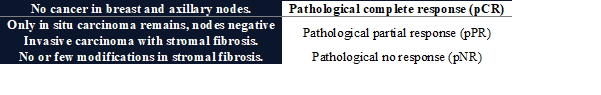
The
overall impact will be assessed by AJCC response criteria (8th edition) (10).
Table 3. AJCC response criteria for overall
impact evaluation.

Results
The
mean age of LABC patients ranged from 35 to 48 years (Figure 1). 57% of
patients had right-sided breast disease. 37 % of patients were postmenopausal
women, and 63% were premenopausal (Figure 2). A positive family history was
found only in one patient, with her mother being affected by the disease.
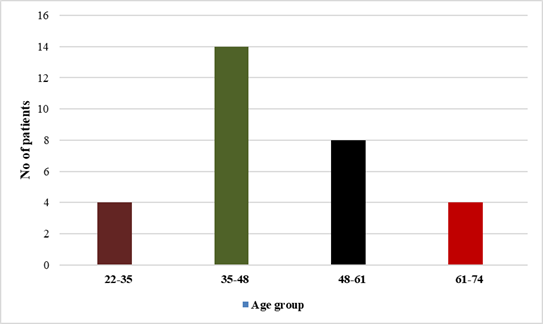
Figure 1. Graph showing
age distribution of LABC patients.
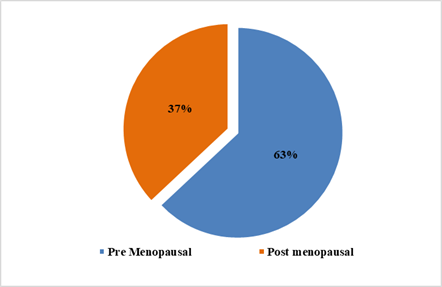
Figure 2. Pie diagram
showing menstrual status of LABC patients.
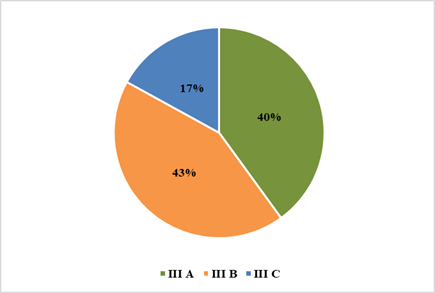
Figure 3. Pie diagram
showing tumour stage.

Figure 4. Graph showing tumour ER, PR, HER2 receptor status.
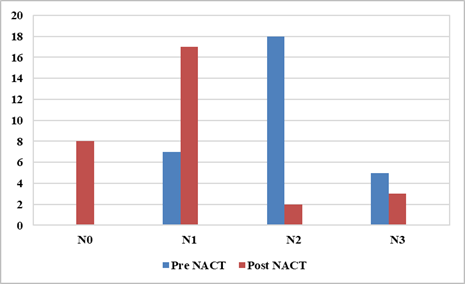
Figure 5. Graph showing
pre NACT vs Post NACT N stage.
Out
of 30 LABC patients, 40% belonged to stage IIIA, 43% were stage IIIB, and 17%
had stage IIIC disease (Figure 3). The percentages of oestrogen
receptor (ER), progesterone receptor (PR), and human epidermal growth factor
receptor 2 (HER2)-positive tumours were 73%, 66%, and
27% respectively (Figure 4). Most of the patients had invasive ductal carcinoma.
Table 4. Pre-NACT T-Stage vs Post-NACT
T-Stage.
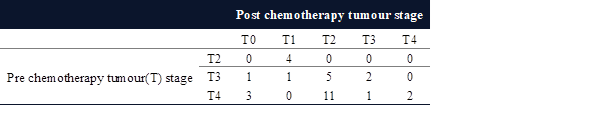
In
our study, most patients belonged to T4b 17 before receiving neoadjuvant
chemotherapy, but post-therapy, the tumour size
reduced, and the majority belonged to T2 17 patients (Table 4). Pre
chemotherapy maximum patients belong to N2 18, but post-therapy maximum
patients belong to N1 17 patients (Figure 5).
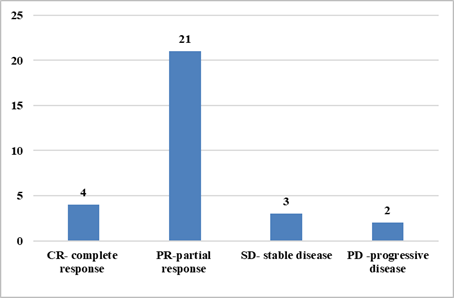
Figure 6. Graph showing
clinical response to NACT.
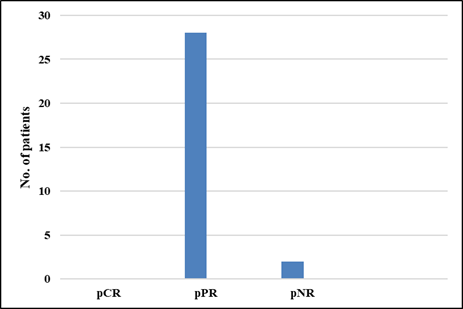
Figure 7. Graph showing
pathological response to NACT.
Figure 8. Graph showing
overall response to NACT.