Giant cell tumor
of the breast masquerading as a malignant breast tumor: a case report
Puja Bhavesh Jarwani 1 *, Virat
Rameshbhai Patel 2, Raval Ravinderkumar Chhabra 1, Vidhyasagar
Sharma 2, Anupama Raval 1
1 Department of Pathology, GCSMCH &
RC, India
2 Department of Surgery, GCSMCH & RC,
India
Corresponding Authors: Puja Bhavesh Jarwani
* Email: pujajarwani@gmail.com
Abstract
Introduction: Giant cell tumours of the soft tissue
(GCT-ST) are usually found in the superficial and deep soft tissues of the
extremities but have been described in the pancreas, lung, thyroid gland,
urothelial tract, skin, larynx, heart and very rarely, in the breast. At
present, according to the World Health Organization's classification of soft
tissue tumors, GCT-ST is categorized as an intermediate grade (rarely
metastasizing) fibrohistiocytic tumour.
GCT of the breast is extremely rare and to date, only eleven cases have been
reported. We report a case of GCT of the breast, which was clinically suspected
as a malignant tumor and discuss the different treatment modalities with the
importance of close follow-up of the same after a thorough review of the
literature.
Case
Presentation: We report a case of a 45-year-old woman who noticed a tender lump in
her left breast. A malignant tumour was suspected on
clinical examination and imaging. Histological evaluation revealed a tumour composed of a mixture of round and oval mononuclear
cells with minimal atypia and uniformly distributed multinucleated
osteoclast-like giant cells (OGCs) with a stroma rich in blood vessels. IHC was
done in which the OGCs stained positively for CD68 and CD45, mononuclear
stromal cells were positive for vimentin whereas the tumour
was negative for breast markers Progesterone Receptor (PR), Estrogen Receptor
(ER), GATA 3, epithelial marker EMA, S-100 and Desmin; hence the definitive
diagnosis of GCT of the breast was made.
Discussion: GCT of the breast, due to its rareness and the malignant-mimicking
clinical presentation, causes difficulty in diagnosis. Other giant cell-rich
lesions including breast cancer with OGCs, pleomorphic leiomyosarcoma,
osteosarcoma, undifferentiated pleomorphic sarcoma and metastatic GCT-B are to
be considered in the differential diagnosis.
Conclusion: GCT of the breast is an extremely rare tumour
and pretends a breast malignant tumours.
For the correct diagnosis of this rare tumour,
combining the results of histological and immunohistochemical analyses helps in
ruling out differential diagnosis
Keywords: Giant cell tumor, Osteoclastic giant cells, Breast,
Immunohistochemistry
Introduction
Giant
cell tumour of soft tissue (GCT-ST) is uncommon and
only a few cases are reported in the medical literature. In 1972, Slam and
Sissons first reported 10 cases of a type of tumour
that originated in the soft tissue but resembled Giant cell tumour
of the bone (GCT-B) in morphology and considered it benign (1). Most GCT-ST
follow a benign clinical course, sometimes locally aggressive, and rarely
metastasize. At present, according to World Health Organization classification
of soft tissues, GCT-ST is categorized as![]() intermediate grade (rarely metastasising) Fibrohistiocytic tumour (2). The histiocytic component is non-neoplastic;
however, they contributes significantly to the
development of the lesion. Although GCT-ST is histologically similar to GCT-B
it has been reported that > 90% of GCT-Bs have a driver mutation in the
H3F3A gene, which makes it distinct from GCT-ST (3). GCT-ST are usually found
in the superficial and deep soft tissues of the extremities but have been
described in the pancreas, lung, thyroid gland, urothelial tract, skin, larynx,
heart and very rarely, in the breast (4). After a comprehensive search of the literature,
we could find only eleven cases of GCT-ST occurring in the breast to date (5-8).
Although GCT-ST is usually considered as an intermediate-grade tumour, the prognosis is uncertain, and the standard
therapy for the GCT of the breast has not been established (9). We report a
case of GCT of the breast, which was clinically suspected as a malignant tumor
and discuss the different treatment modalities with the importance of close follow-up
of the same after a thorough review of the literature.
intermediate grade (rarely metastasising) Fibrohistiocytic tumour (2). The histiocytic component is non-neoplastic;
however, they contributes significantly to the
development of the lesion. Although GCT-ST is histologically similar to GCT-B
it has been reported that > 90% of GCT-Bs have a driver mutation in the
H3F3A gene, which makes it distinct from GCT-ST (3). GCT-ST are usually found
in the superficial and deep soft tissues of the extremities but have been
described in the pancreas, lung, thyroid gland, urothelial tract, skin, larynx,
heart and very rarely, in the breast (4). After a comprehensive search of the literature,
we could find only eleven cases of GCT-ST occurring in the breast to date (5-8).
Although GCT-ST is usually considered as an intermediate-grade tumour, the prognosis is uncertain, and the standard
therapy for the GCT of the breast has not been established (9). We report a
case of GCT of the breast, which was clinically suspected as a malignant tumor
and discuss the different treatment modalities with the importance of close follow-up
of the same after a thorough review of the literature.
Case report
A 45-year-old married woman was referred to the General Surgery
Department after she noticed a painful lump in her left breast. The patient had
no history of trauma, no significant family history and had achieved menarche at
at the age of 14 with regular menstrual cycle of 3-4 days. Obstetric
History: G4P4A3L1. Physical examination revealed a 4 x 4 cm hard, painful lump
in the upper inner quadrant of the left breast. The contralateral breast was
unremarkable. There was no palpable axillary, supraclavicular or
infraclavicular lymph node. Malignancy was strongly suspected on clinical
examination. Ultrasound showed approximately 30 x 25 mm sized well definedmixed echogenic lesion with internal cystic areas in
upper inner quadrant at 9’o clock position in left breast. Mammography
indicated a hyperdense BIRAD- IV lesion (suspicious for malignancy) (Figure 1).
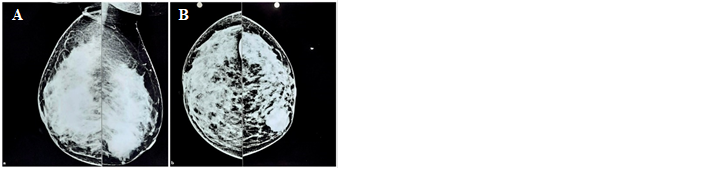
Figure 1. Mammography of Bilateral Breasts showing a hyperdense BIRADS IV lesion,
suspicious for malignancy. A. Mediolateral view, B. Craniocaudal view.
Fine needle aspiration cytology (FNAC)
correlation was advised. FNAC examination showed numerous multinucleated giant
cells with foci of stromal cells and was suggestive of Giant cell-rich
fibroepithelial lesion. Trucut biopsy from the lesion
showed numerous multinucleated giant cells with stroma showing minimal atypia
and low mitoses. No ductal element was seen. Findings were suggestive of Giant
cell rich stromal lesion. Excision of the lesion was performed and sent for
histopathological examination. Gross examination showed the presence of breast
tissue with tumour total measuring 7.5x6.2x2.5cm. The
outer surface was smooth, multilobulated and covered with fibro fatty tissue.
On cutting, there was the presence of one well-circumscribed, solid cystic tumour measuring 6.0x4.0x2.0cm. The Cut surface showed a firm
greyish-white solid area measuring 4.0x2.5x1.3cm along with a few hemorrhagic
and cystic areas.
Histological evaluation of
the tumour revealed a mixture of round to oval
mononuclear stromal cells with minimal atypia and uniformly distributed
multinucleated osteoclast-like giant cells (OGCs). Stroma was rich in blood
vessels and showed marked hyalinization along with focal metaplastic bone
formation. Surrounding breast parenchyma showed fibrocystic changes. There was
no evidence of granuloma formation.
(Figure 2A: H& E stain, x100. The tumour
revealed a mixture of round and oval mononuclear cells and uniformly
distributed multinucleated osteoclast-like giant cells and Figure 2B: H& E stain,
x400. Mononuclear stromal cells show minimal atypia and scanty mitoses). For
further evaluation, sections were subjected to immunohistochemistry examination
(IHC). The giant cell component of the tumour showed
a strong positive reaction to histiocytic marker CD68 (Figure. 2C: IHC stain
for CD68, x400. Osteoclastic giant cells stained positive for CD68). They also
gave a positive reaction to leucocyte common antigen CD45. The stromal
mononuclear component was stained positive for mesenchymal marker vimentin (Figure.
2D: IHC stain for Vimentin, x400. Mononuclear stromal cells stained positive
for vimentin). The tumour was negative for breast
markers like Progesterone Receptor (PR), Estrogen Receptor (ER) and GATA 3
along with Epithelial membrane antigen (EMA) (Figure. 2E: IHC stain for EMA,
x400. Tumor cells negative for EMA). These findings along with relatively
homogeneous bland-appearing features throughout the whole tumour
ruled out breast cancer with OGCs. Lack of pleomorphic cells and IHC negative
for Desmin ruled out pleomorphic leiomyosarcoma. The absence of sarcomatous
features and malignant osteoid formation ruled out osteosarcoma. Although the tumour cells were positive for vimentin and negative for
EMA, S-100 and Desmin, the absence of pleomorphic spindle cells and atypical
mitoses ruled out the diagnosis of undifferentiated pleomorphic sarcoma. As
there was no history of GCT of the bone, metastatic GCT of the bone was ruled
out. The Ki-67 proliferation index was 20-25% in the stromal cells (Figure. 2F:
IHC stain for Ki-67, x400. Ki-67 was positive in 20-25% of stromal tumor
cells).
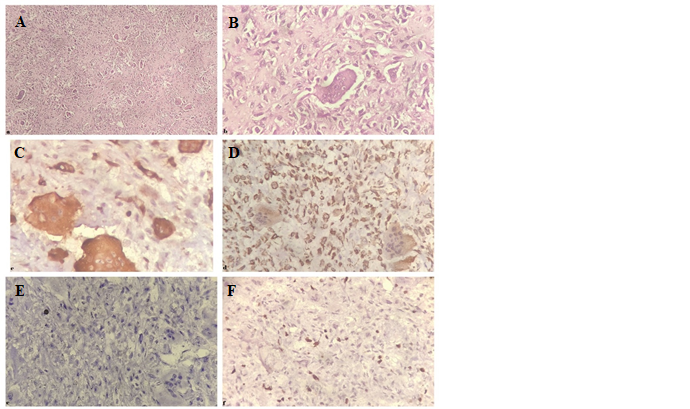
Figure 2. A.
H& E stain, x100. The tumour revealed a mixture
of round and oval mononuclear cells and uniformly distributed multinucleated osteoclast-like
giant cells. B. H& E stain, x400. Mononuclear stromal cells show
minimal atypia and scanty mitoses. C. IHC stain for CD68,
x400.Osteoclastic giant cells stained positive for CD68. D. IHC stain
for Vimentin, x400. Mononuclear stromal cells are positive for vimentin. E. IHC
stain for EMA, x400. Tumor cells negative for EMA. F. IHC stain for
Ki-67, x400. Ki-67 was positive in 20-25% of tumor cells.