Papillary thyroid
carcinoma arising from mature cystic teratoma ovary: a case report
Praveen Jacob Ninan 1, Nimitha Nizar 1,
V S Haritha 1*
1 Department of Radiation Oncology, Government T.D.
Medical College, Alappuzha, Kerala, India
Corresponding Authors: V
S Haritha
* Email: vsharitha26@gmail.com
Abstract
Introduction: Mature cystic teratoma is a kind of ovarian germ cell tumour.
Malignant transformation in it is uncommon with thyroid cancer being rarely
found. Given its rarity and nonspecific symptoms, misdiagnosis and indifference
when compared to other ovarian lesions is very common.
Case presentation: Herein we report a
case of a 58-year-old post-menopausal female who presented with a history of
abdominal distension and loss of appetite. She was found to have an
abdominopelvic mass on examination and a raised CA125 levels for which she
underwent an MRI pelvis which was suggestive of an O-RADS 5 lesion for which
she underwent a staging laparotomy. The final histopathology and
immunohistochemistry were suggestive of papillary thyroid carcinoma arising
from a mature ovarian teratoma. After a multidisciplinary tumour board
analysis, she was planned to be kept under follow–up with regular serum
thyroglobulin monitoring. She has no signs of disease recurrence to date.
Discussion: Struma ovarii is one type of monodermal ovarian teratoma in which the
tumour contains more than 50 % thyroid tissue. Diagnosis in such cases is
difficult due to the lack of typical symptoms. In most of the cases, the
diagnosis is incidental. Optimal treatment is still unclear given the rarity of
the disease. In a few cases, thyroidectomy was done whereas in a few others it
was omitted. Further therapy may include radioiodine treatment if needed..
Conclusion: To the best of our knowledge there is very scant information available
on the natural history, prognosis and management of papillary thyroid carcinoma
arising from mature cystic teratoma ovary. Hence, a multidisciplinary treatment
approach may be needed for the same.
Keywords: Papillary thyroid carcinoma, Mature cystic teratoma, Germ cell tumour
Introduction
Mature cystic teratomas comprise 20% of all ovarian
neoplasms and are considered to be the most common type of germ cell tumors of
the ovary (1). They can be either unilateral or bilateral and commonly appear
in reproductive age, but have also been reported in postmenopausal women and
children (2). Malignant transformation is uncommon, with an estimated risk of
0.17% to 2% (3). When malignant transformation occurs, in most cases (80%) it is squamous cell carcinoma as histology
(4). Less common ones include sarcomas, adenocarcinomas, malignant melanomas,
basal cell carcinomas, carcinoid tumors, and thyroid carcinomas (5). Struma
ovarii is a rare ovarian lesion that is characterized by the presence of
thyroid tissue in at least half of the overall ovarian mass. This mass comprises less than 1 % of
ovarian tumors and also upto 2 to 5 % of all ovarian teratomas. The patients
usually are asymptomatic with pelvic mass and pain being the common presenting
symptoms, making it usually diagnosed post-operatively based on histopathology
(6). A small proportion of struma ovarii may undergo malignant transformation,
with papillary carcinoma the most common type of malignancy seen. The criteria
used to identify a malignant change in struma ovarii are identical to those
used to evaluate the thyroid gland (7). Only 5–8 % of these patients
usually have clinical hyperthyroidism (8). Owing to the rarity of the tumor,
there are no specific clinical, radiological, or serum markers that distinguish
struma ovarii in the absence of thyroid hormone abnormalities. Thus, a
definitive diagnosis is made by histopathological examination (7). Herein we
present a case of papillary thyroid carcinoma arising within a mature cystic
ovarian teratoma in a 58 year old post menopausal female.
Case presentation
A 58 year old post menopausal female presented with a
two months history of abdominal distension and loss of appetite. On examination
she had a palpable mass per abdomen which was of 18 week size felt more towards
the left side . An ultrasonography of the abdomen was done which revealed a
large abdominopelvic multilocular cystic lesion which was likely of pelvic origin . An MRI pelvis followed which revealed
a large abdominopelvic cystic lesion of 12.8 x11.7x 15.2 cm with multiple
internal septation (Ovarian-Adnexal Reporting and Data System - O- RADS 5 )
with mild ascites and no evidence of pelvic lymphadenopathy (Figure1).
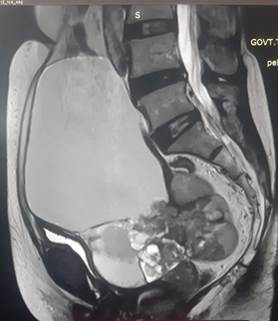
Figire 1. MRI pelvis showed an abdominopelvic cystic
lesion.
Her serum
tumour markers showed a raised CA – 125(Cancer Antigen 125) level (157.8 U/L). Serum CEA(
Carcinoembryonic Antigen) and CA19.9 (Cancer Antigen 19.9 ) were within the normal
limits. She then underwent a total abdominal hysterectomy with bilateral
salpingoophorectomy with omental biopsy. Intraoperatively she was found to have
a large cystic lesion of around 15x 15 cm replacing the whole of the left ovary
with smooth surface and no papillary excruscences. The right ovary was adherent
to the mass and uterus was found to be atrophic . The omentum was found to have
nodularity and a biopsy was taken from it and ascitic fluid was sent for
cytology. The pathology findings showed an ovarian mass of size 19x13x 8 cm
with the bosselated surface which was solid and cystic with multiple rents on
the surface. The microscopic examination showed papillary carcinoma with no
immature component (Figure 2).
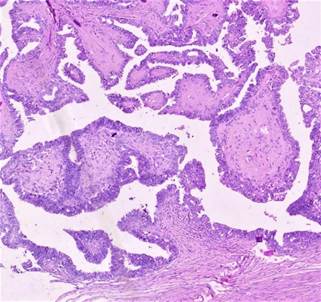
Figure 2. Histopathology image [40 X magnification]
showing papillary thyroid carcinoma in a mature cystic ovarian teratoma.
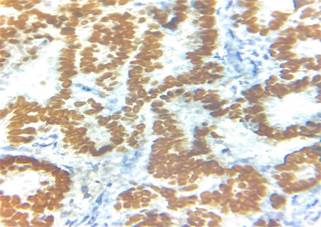
Immunohistochemistry showed diffuse strong
positivity for PAX8 ( Paired Box 8), TTF 1(Transcription Termination
Factor 1), thyroglobulin (Figure 3) and negativity for WT1 (Wilms' tumour gene
1 ) thus confirming the final
diagnosis of papillary thyroid carcinoma in a mature cystic ovarian teratoma.
The omental biopsy was suggestive of congestion only. Ascitic fluid cytology
was doe which was found to be negative. Thyroid function tests were done which were
found to be normal. Serum alpha-fetoprotein [AFP] was found to be 7.46 and beta
HCG (human chorionic gonadotrophin) to be 5.55. Post-operative CA-125 was 13.9
U/L. An ultrasound neck was done which showed a small solid nodule measuring
2.7 x 1.7 mm in the midpole of right lobe with no calcification. A fine needle
aspiration was done which was negative for malignancy. She was planned to be
kept on follow–up with serum thyroglobulin check every 6 months. At present,
she has completed about 6 months of follow-up with no signs of disease
recurrence anywhere.
![]()
![]()
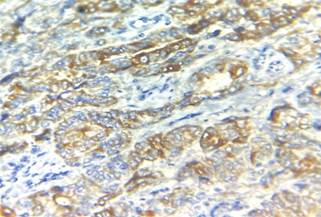
Figure 3. Immunohistochemistry images showing TTF-1
and thyroglobulin positivity.
Discussion
Struma ovarii is an ovarian germ cell tumour. It
comprises of more than 50% thyroid tissue and can be differentiated from a
mature teratoma, which contains only a small component (less than 50%) of
benign thyroid tissue. Struma ovarii typically arises unilaterally, with 5% of
cases seen bilaterally. A small proportion of struma ovarii may undergo
malignant transformation (7). Malignant struma ovarii was first described by
Wetteland in 1956 (9). Malignancy in struma ovarii is diagnosed based on
histopathological criteria and guidelines for primary thyroid gland disease.
Papillary and follicular carcinoma are the common histologies seen (10)0
Differentiated thyroid carcinoma arising from an MCT is rare with an estimated
incidence being that of 0.1% to 0.2% (8).
It is typically found incidentally in histopathology (5).
Multiple molecular abnormalities have been reported in
thyroid cancer arising from ovarian teratomas, primarily in malignant struma
ovarii. In thyroid carcinomas arising within MCT without struma ovarii, no
molecular markers have been reported. Molecular genetics may help to
differentiate benign from malignant lesions. However, it is uncertain if they
have a significant impact on cancer prognosis in this type (5).
Struma ovarii
may mimic the clinical symptoms of ovarian malignancy, presenting with ascites,
a complex ovarian cyst, and an elevation of CA-125 (7). A case of pseudo-Meigs
syndrome which includes ascites in the setting of hydrothorax, and elevated CA
125 levels has been described in malignant struma ovarii. The associated
symptoms disappear, and the elevated CA 125 levels return to normal postoperatively
usually without adjuvant therapy (11). Metastasis of malignant struma ovarii is
seen in approximately 5 to 23 % of cases and is mainly intra-abdominal,
although blood-borne metastasis can occur in the liver, lung, brain, bone,
vertebra, and the contralateral ovary (8). Follicular carcinoma is more likely
to metastasize to the lung, liver, and central nervous system whereas papillary
carcinoma is said to involve the abdominal cavity and lymph nodes and
occasionally the liver (12).
Dane et al (13) reviewed 15 cases of
differentiated thyroid carcinoma arising in a mature ovarian teratoma and since
then, 4 additional cases have been reported. (14 -17) Most patients, as in our
case, presented with abdominal pain, only 2 patients did not report any
symptoms. Papillary thyroid carcinoma (PTC) was the most common histopathologic
type (53%), followed by follicular variant of PTC (42%) and follicular
carcinoma (5%). Only 2 cases presented with thyroid tumor size ⩽1 cm (5).
Ryder et al (18) reported a 0.9-cm
follicular variant PTC within a 4.6-cm mature cystic teratoma (MCT). Thyroid
ultrasound and 131I diagnostic whole body scan were normal. No
further treatment was performed on this patient. Dias et al (17)
reported 2 foci of follicular variant PTC (the largest of 3 mm) within a 4.5-cm
mature ovarian teratoma. Thyroid ultrasound was also normal and no additional
treatment was done.
The optimal treatment of thyroid carcinoma arising
within MCT is unclear because of the rarity of the disease. Moreover, no data
on recurrence are available. In some of the reported cases, thyroidectomy was
performed (5). whereas in some others, no thyroidectomy was performed ( 19-21).
In these cases, no primary thyroid carcinoma was clinically apparent in further
follow-up.
Differentiated thyroid carcinomas seen in struma
ovarii can rarely present as a locally invasive or metastatic disease (22).
Ovarian metastases from a primary thyroid carcinoma may occasionally occur and
in such cases, the ovarian mass does not present with teratomatous
characteristics (23).
After surgical resection subsequent therapy depends on
the extent of the primary lesion and disease stratification. There is no
consensus on the optimal treatment of malignant struma ovarii. Treatment
recommendations are based on either single case reports or case series. Further
therapy may include total thyroidectomy and radioiodine ablation which needs
thyroglobulin monitoring, as well as radioiodine treatment if needed (24).
Conclusion
To our best knowledge, there is very scant information
on the natural history and prognosis of papillary thyroid carcinoma arising on
a mature cystic ovarian teratoma. Currently, there is no management consensus on
this entity. It is important to have a multidisciplinary approach in such cases
with an individualized approach toward treatment. We believe that a long-term
follow-up is needed to comment on the natural course and prognosis of this
disease.
Author contribution
PJN, NN,
and VSH contributed to the conception, design, and definition of intellectual content, literature search, data
acquisition, data analysis, statistical analysis, manuscript preparation,
editing and review.
Conflict of interest
The
authors declare no conflict of interest.
References
1.
Yassa L, Sadow P, Marqusee E. Malignant struma ovarii. Nat Clin
Pract Endocrinol Metab 2008;4:469–72.
2.
Hasleton PS, Kelehan P, Whittaker JS, et al. Benign and
malignant struma ovarii. Arch Pathol Lab Med 1978;102:180–4.
3.
Curling OM, Potsides PN, Hudson CN. Malignant change in benign cystic
teratoma of the ovary. Br J Obstet Gynaecol. 1979;86:399–402
4.
Sakuma M, Otsuki T, Yoshinaga K, et al. Malignant transformation arising
from mature cystic teratoma of the ovary: a retrospective study of 20
cases. Int J Gynecol Cancer. 2010;20:766–771.
5.
Pineyro, M. M., Pereda, J., Schou, P., de Los Santos, K., de la Peña,
S., Caserta, B., & Pisabarro, R. (2017). Papillary Thyroid Microcarcinoma
Arising Within a Mature Ovarian Teratoma: Case Report and Review of the Literature. Clinical
medicine insights. Endocrinology and diabetes, 10, 1179551417712521.
6.
Zamani, F., Abdolrazaghnejad, A., Ameli, F., GHashghaee, S., Nassiri,
S., & Zamani, N. (2022). Struma ovarii: A case report and review the
literature. International journal of surgery case reports, 96,
107318.
7.
Rahma, A., Mardiyana, L., & Fauziah, D. (2022). Malignant struma
ovarii: Case report of an unusual ovarian tumor with CT imaging. Radiology
case reports, 17(5), 1705–1708.
8.
Makani K. Gaba. Struma ovarii with a focus of papillary thyroid. Gynecol
Oncol. 2004;94(3):835–839. doi: 10.1016/j.ygyno.2004.06.003.
9.
Wetteland. Malignant struma ovarii [in Norwegian]. Nord Med.56(43).
1956; 56(43): p. 1568–1570.
10.
Kumar SS, Rema P, R AK, Varghese BT. Thyroid type papillary carcinoma
arising in a mature teratoma. Indian J Surg Oncol. 2014 Sep;5(3):168-70.
11.
Zannoni , Gallotta , Legge , Tarquini , Scambia , Ferrandina.
Pseudo-Meigs’ syndrome associated with malignant struma ovarii: a case report.
Gynecol Oncol. 2004; 94(1).
12.
Roth K. Highly differentiated follicular carcinoma arising. Int
J Gynecol Pathol. 2008;27(2):213–222.
13.
Dane C, Ekmez M, Karaca A, Ak A, Dane B. Follicular variant of papillary
thyroid carcinoma arising from a dermoid cyst: a rare malignancy in young women
and review of the literature. Taiwan J Obstet Gynecol. 2012;51:421–425.
14.
Cymbaluk-Ploska A, Chudecka-Glaz A, Chosia M, Ashuryk O, Menkiszak J. Conservative
treatment of a young patient with thyroid carcinoma in adult ovarian
teratoma—case report. Gynecol Endocrinol. 2014;30:187–191
15.
Uzum AK, Iyibozkurt C, Canbaz B, et al. Management and follow-up results
of an incidental thyroid carcinoma in a young woman with ovarian
teratoma. Gynecol Endocrinol. 2013;29:724–726.
16.
Souaf I, El Fatemi H, Bennani A, et al. Papillary carcinoma derived from
ovarian mature cystic teratoma: a new case report and literature review. Case
Reports Clin Med. 2014;3:197–202.
17.
Dias G, Diniz da Costa T, Pedro A, Silva Pereira J. A case of follicular
variant of papillary thyroid carcinoma in a mature cystic teratoma in a young
woman. Acta Obs Ginecol Port. 2015;9:302–304.
18.
Ryder M, Nikiforov YE, Fagin JA. Follicular variant papillary thyroid
carcinoma arising within an ovarian teratoma. Thyroid. 2007;17:179–180.
19.
Doldi N, Taccagni GL, Bassan M, et al. Hashimoto’s disease in a
papillary carcinoma of the thyroid originating in a teratoma of the ovary
(malignant struma ovarii) Gynecol Endocrinol. 1998;12:41–42.
20.
Lee JM, Kim JW, Song JY, et al. Adenocarcinoma arising in mature cystic
teratoma: a case report. J Gynecol Oncol. 2008;19:199–201
21.
Lataifeh I, Abdel-Hadi M, Morcos B, Sughayer M, Barahmeh S. Papillary
thyroid carcinoma arising from mature cystic teratoma of the ovary. J
Obstet Gynaecol (Lahore) 2010;30:884–886.
22.
Zhu Y, Wang C, Zhang G-N, et al. Papillary thyroid cancer located in
malignant struma ovarii with omentum metastasis: a case report and review of
the literature. World J Surg Oncol. 2016;14:17
23.
Brogioni S, Viacava P, Tomisti L, Martino E, Macchia E. A special case
of bilateral ovarian metastases in a woman with papillary carcinoma of the
thyroid. Exp Clin Endocrinol Diabetes. 2007;115:397–400
24.
Goffredo P, Sawka AM, Pura J, Adam MA, Roman SA, Sosa JA. Malignant
struma ovarii: a population-level analysis of a large series of 68
patients. Thyroid. 2015;25:211–215.