Biological
properties and therapeutic effects of apigenin and its evaluation on several
types of cancer
Roshanak Ale-Esmaiel 1, Seyyed Mohammad Taghi
Razavi-Toosi 2,3*
1 Radiology and Nuclear Medicine Department, School of
Paramedical Sciences, Kermanshah University of Medical Sciences, Kermanshah,
Iran
2 Medical
Biotechnology Research Center, School of Paramedicine, Guilan University of Medical
Sciences, Rasht, Iran
3 Department of Physiology, Faculty of Medicine, Guilan
University of Medical Sciences, Rasht, Iran
Corresponding Authors: Seyyed
Mohammad Taghi Razavi-Toosi
* Email: Smtrazavi@gums.ac.ir
Abstract
Apigenin is a member of the flavonoid family that has been used in
medicine for a long time. Apigenin is one of the compounds that has been used
for a long time to treat various disorders and diseases. Apigenin is chemically
known as 4',5,7, trihydroxyflavone and belongs to the family of flavones.
Apigenin has many pharmacological activities such as anti-inflammatory,
anti-viral, anti-bacterial, etc. Various studies have shown that apigenin plays
an important role in suppressing diseases such as Parkinson's, Alzheimer's,
inflammatory diseases, and different types of cancers. In the present study,
various therapeutic properties, biological effects, and the effect of apigenin
on different cancers are discussed. Different studies have been conducted on
the anti-cancer effect of apigenin. It has been proven that apigenin has
inhibitory effects on various cancers including lung, stomach, neuroblastoma,
thyroid, liver, skin, and prostate cancer through different signaling pathways.
In general, it can be mentioned that the anti-cancer properties of apigenin are
due to its effects in various signaling pathways such as angiogenesis, tumor
suppressor genes, apoptosis, cell cycle and nuclear factor kappa B (NF-κB),
Janus kinase/signal transducer and activator of transcription (JAK/STAT3),
phosphoinositide 3-kinase /protein kinase B /mammalian target of rapamycin
(PI3K/AKT/mTOR), mitogen-activated protein kinase/ estrogen receptor 2
(MAPK/ER2), Wnt/B-catenin pathways.
Keywords: Inflammation, Apigenin, Cancer, Cell cycle, Apoptosis
Introduction
Polyphenol
compounds are described by phenolic structures. These biological molecules are
used in the treatment of various diseases. They are a large family of natural
compounds that have many biological, pharmacological, and physiological
advantages for human health. They are known as protectors against oxidative
stress, ultraviolet and other pathogens. Polyphenols can play their role in
cell protection against oxidative stress and inflammation by activation of the
transcription factor nuclear factor erythroid-2 related factor (Nrf2) (1-5). In addition,
these compounds can modulate some of the most important cellular processes such
as proliferation, cell growth, differentiation, etc. (6). Various studies have shown that
polyphenols are effective in radiation protection. The main mechanisms are
neutralizing free radicals caused by radiation, reducing inflammatory
responses, repairing hematopoietic cells, and repairing deoxyribonucleic acid
(DNA) (7). So far, more than 8000
polyphenolic compounds have been known in different plants. Polyphenols have
different chemical structures, the most prominent of which are flavonoids,
stilbenes, and phenolic acids. One of the most important polyphenolic compounds
is flavonoids (1, 3, 8, 9). The first
studies on flavonoid compounds were done in 1936. Flavonoids are low molecular
weight compounds. Although flavonoids are not made by humans and animals, they
are considered essential compounds in the human diet. The compounds are
abundant in our diet, including nuts, fruits, flowers, seeds, stem, wine, and
tea (10, 11). Flavonoids are divided into
different classes according to their molecular structures such as flavanones,
flavones, flavanols, isoflavones, flavanonols, neoflavanes, flavanes, and
flavonols (10, 12). Flavonoids are almost 5000
compounds that chemically have a prevalent phenylchromanone structure
(C6-C3-C6). The general structure of flavonoid is based on two benzene rings (A
and B ring) that are connected by a heterocyclic pyran (C ring) that contains
oxygen. They have indicated various biological effects such as
anti-inflammatory, antiviral, anti-mutagenic, and free radical scavenging (11, 13, 14). One of the
flavonoids that have attracted a lot of attention is apigenin (15). Apigenin is one of the sub-classes
of flavones, the unique properties of flavones are non-toxic and non-mutagenic
(Figure1). Apigenin is mainly found in fruits (oranges), vegetables (onion,
parsley, celery), herbs (basil, oregano, thyme, chamomile), and in some
seasonings (13, 16, 17). Table 1
demonstrates common plants contain the highest amount of apigenin. Apigenin is
chemically represented as 4',5,7, trihydroxyflavone. It's a low molecular
weight flavonoid (270.24=KDa). In general, apigenin is insoluble in water, but
the best solvents for this substance are dilute dimethyl sulfoxide (DMSO),
potassium hydroxide (KOH), dimethylformamide (DMF), and ethanol (12, 13, 20,
21). Its melting point is reported as 347.5. The pure form of apigenin is
unstable and is usually recommended to be kept at -20oC (13). For a long time,
apigenin has been used to treat various diseases, including insomnia,
Parkinson's, asthma, nervous system disorders, indigestion, gastritis, cancers,
and cardiovascular diseases (12, 13, 22). Apigenin can also modulate different
intercellular and extracellular signaling pathways to prevent abnormal tissue
growth. For this reason, the administration of apigenin can be one of the
effective factors in cancer treatment (22). Although previous studies have
indicated that flavonoid compounds cannot have a good effect on blood lipid
metabolism, apigenin plays a considerable role in regulating blood lipid and
reduces triglyceride, cholesterol, and low-density lipoprotein cholesterol in
the serum of mice (23). As a result, apigenin has attracted a lot of attention
due to its low toxicity and significant impacts on natural versus cancer cells
compared to other flavonoids (24). In this article, the biological effects of
apigenin are discussed first, and then the effect of this flavonoid compound on
several cancers is investigated. It has been reported that apigenin and other
medicinal herbs can have remarkable effects in preventing various diseases and
cancers. And also, it has been shown that different phytochemicals including
flavonoids are responsible for the therapeutic impacts of these plants (25).
Various studies have demonstrated that apigenin has different biological
effects such as anti-inflammatory, anti-carcinogenic, anti-mutagenic,
antioxidant, anti-viral, anti-allergic cardioprotective, neuroprotective, and
antibacterial. In general, it can be said that apigenin has attracted more
attention due to its considerable effects on cancers and low toxicity compared
to other flavonoids (13, 15, 24, 26, 27). The biological properties mentioned
above are caused by the functional mechanisms of flavonoid compounds such as
apoptosis induction, stimulation of the immune system, improvement of the
enzymatic detoxification activity, reduction of oxidative stress, and cell
cycle inhibition (15, 28). Some of these biological effects of apigenin are
discussed below.
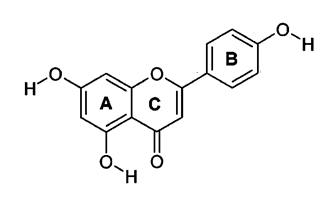
Figure 1. The basic
structure of apigenin.
Table 1. Plants with the highest level of apigenin.
|
Scientific name |
Commonly known |
|
Achillea millefolium |
Yarrow |
|
Apium graveolens |
Celery |
|
Artemisia dracunculus |
Tarragon |
|
Chamaemelum nobile |
Perennial
chammomile |
|
Coriandrum sativum |
Cilantro |
|
Digitalis purpurea |
Purple
foxglove |
|
Echinacea spp |
Coneflower |
|
Gingko biloba |
Biloba |
|
Glycyrrhiza glabra |
Licorice |
|
Linum usitatissimum |
Flax |
|
Marrubium vulgare |
Horehound |
|
Matricaria retcutita |
Annual
chamomile |
|
Mentha spicata |
Spearmint |
|
Ocimum basilicum |
Basil |
|
Origanum vulgare |
Oregano |
Anti-inflammatory effects of apigenin
Inflammation is a critical immune response to maintain tissue
homeostasis. Two different types of inflammation are acute and chronic
inflammation. Acute inflammation is a protective and essential response of
therapeutic processes that initiates rapidly and its symptoms last for a short
period up to a few days. In general, this response should be local and limited. Although
acute inflammation tries to restore homeostasis, if it is not resolved, it
leads to chronic inflammation (18). And also, Inflammation is one of
the most important characteristics that confirm tumor progression and increase
the risk of cancer. Flavonoids such as apigenin have been shown to suppress the
activation of different cytokines and immune cells, so they may be considered
natural inhibitors that can stop the activation of an adaptive and innate
immune system. Apigenin can diminish inflammation by inhibiting tumor necrosis
factor-alpha (TNF-![]() ), C-C motif
chemokine ligand 2 (CCL-2), granulocyte-macrophage colony-stimulating factor
(GMCSF), interleukin 1-alpha (IL-1
), C-C motif
chemokine ligand 2 (CCL-2), granulocyte-macrophage colony-stimulating factor
(GMCSF), interleukin 1-alpha (IL-1![]() ) and IL-6 (19, 20). Many studies have indicated that
apigenin can enhance various anti-inflammatory pathways, such as
phosphatidylinositol 3-kinase/ protein kinase B (PI3k/Akt) and p38
mitogen-activated protein kinase (P38/MAPK). The inflammatory and antioxidant
pathways of apigenin in cell lines are shown in figure 2. In addition, it
reduces the activity of nitric oxide synthase-2 and ccyclooxygenase-2 (Cox-2).
And also, apigenin prevents TNF-
) and IL-6 (19, 20). Many studies have indicated that
apigenin can enhance various anti-inflammatory pathways, such as
phosphatidylinositol 3-kinase/ protein kinase B (PI3k/Akt) and p38
mitogen-activated protein kinase (P38/MAPK). The inflammatory and antioxidant
pathways of apigenin in cell lines are shown in figure 2. In addition, it
reduces the activity of nitric oxide synthase-2 and ccyclooxygenase-2 (Cox-2).
And also, apigenin prevents TNF-![]() -induced
nuclear factor kappa B (NF-κB) activation and IkappaB kinase degradation (21). Apigenin can exert a wide range of
molecular signaling effects (22). It has been reported that apigenin
inhibits mitogen-activated protein kinase (MAPK) and the activity of protein
kinase-C (23, 24). On the other hand, apigenin is a
famous protein-tyrosine kinase inhibitor. In addition, it has been indicated
that it can inhibit extracellular signal-regulated
kinases (ERK) (25). Inactivation of NF-κB by apigenin
in human cells culture medium is through suppression of the phosphorylation of
the p65 subunit (26).
-induced
nuclear factor kappa B (NF-κB) activation and IkappaB kinase degradation (21). Apigenin can exert a wide range of
molecular signaling effects (22). It has been reported that apigenin
inhibits mitogen-activated protein kinase (MAPK) and the activity of protein
kinase-C (23, 24). On the other hand, apigenin is a
famous protein-tyrosine kinase inhibitor. In addition, it has been indicated
that it can inhibit extracellular signal-regulated
kinases (ERK) (25). Inactivation of NF-κB by apigenin
in human cells culture medium is through suppression of the phosphorylation of
the p65 subunit (26).
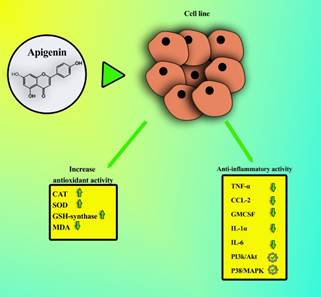
Figure 2. Anti-inflammatory and antioxidant
effects of apigenin. Apigenin decreases malondialdehyde (MDA), increases
antioxidants enzymes such as catalase (CAT), superoxide dismutase (SOD),
glutathione synthetase (GSH-synthase), reduces the activity of anti-inflammatory
cytokines, including tumor necrosis factor-alpha (TNF-α), c-c motif chemokine
ligand 2 (CCL-2), granulocyte-macrophage colony-stimulating factor (GMCSF), interleukin 1-alpha (IL-1α), interleukin 6 (IL-6), and
also it can can promote different anti-inflammatory pathways, such as
phosphatidylinositol 3-kinase/ protein kinase B (pI3k/Akt) and p38
mitogen-activated protein kinase (p38/MAPK).
The
effect of apigenin on the cell cycle
Cell
division activates cell proliferation and distributes the exact genetic copies
to daughter cells which is essential for the reproduction of life (27). Studies have demonstrated that
medicinal plants can have a considerable role in cell cycle arrest, which is
done by inhibiting G0/G1 or G2/M checkpoints. Several biochemical events cause
cells to progress through the cell cycle. Before cells enter the S phase, a
cascade of events must occur, including in the level of D-type cyclins and
cyclin E at the beginning and the end of G phase. In general, with the
formation of D-type cyclins complex with cyclin-dependent kinase 2 and CDK 4,
cell cycle progress occurs through phosphorylation tumor suppressor protein
retinoblastoma (Rb), which is necessary to enter the S phase. Apigenin is known
as an effective inhibitor of some protein tyrosine kinases such as steroid
receptor coactivator (Src) tyrosine kinase and epidermal growth factor
receptor. Apigenin also can suppress the activation of protein kinase B/AKt,
phosphatidylinositol 3-kinase, and casein kinase-2, which can play an important
role in the development of cancer. In fact, apigenin has been indicated to inhibit
cyclin-dependent kinases (CDKS) and cyclins in vitro. In addition, apigenin can
enhance CDK inhibitors such as KIP1/p17 and WAF1/p21, which reduces the
activity of G1 CDK, p53 stabilization, and Rb dephosphorylation (39). In one of
the studies, the inhibitory effect of apigenin on the growth of human prostate
tumor cells was evaluated in nude mice. In this study, apigenin was
administrated orally. The consumption of apigenin increased the expression of
WAF1/p21, KIP1/p27, INK4c/p18 and INK4a/p16, decreased the expression of
cyclins D1, D2, E; and cyclin-dependent kinase
(CDK), including CDK2 and CDK4 (Figure 3). With the decrease of cyclin D1, the
inhibitor of WAF1/p21 increases. On the other hand, CDK4 can be partially
reduced while cyclin E remains unchanged. These findings show that the
inhibitory effect of apigenin on the proliferation of cells in the G1 phase is
due to its decrease of cyclins D1 and the increase of WAF1/p21.Another
mechanism of cell growth inhibition by apigenin has also been investigated.
When cells are exposed to apigenin, the amount of protein p53 and its
downstream proteins, such as Protein p21(Cip1/Waf1), which is a potential CDK
inhibitor in G1 and G2/M phases, increases and leads to the inhibition of the
cell cycle (28, 29). As mentioned above, apigenin
causes cell cycle arrest in different phases such as G1/S or G2/M, which is
done by modulating the expression of CDKs and other related genes (30). It has been indicated that
exposure to a broad range of malignant cells such as fibroblast and epidermal
cells with apigenin causes a reversible G0/G1 and G2/M arrest through the
inhibition of p53 (CDK2) kinase activity along with enhancement of the stability
of the p53 protein (31, 32).

Figure 3. The above image
shows the effects of apigenin on influencing factors in cell cycle. Apigenin
can inhibit the cell cycle by increasing the expression of WAF1/p21, KIP1/p27,
INK4c/p18 and INK4a/p16, (Cip1/Waf1), p53, and decreasing the expression of
cyclins D1, D2, E; and cyclin-dependent kinase, including CDK2 and CDK4.
The
effect of apigenin on apoptosis
Different
types of cell death are necrosis, apoptosis, pyroptosis, and autophagy.
Programmed cell death is an essential process in multicellular organisms that
removes hazardous cells and keeps tissue homeostasis. Apoptosis is one of the
types of regulated cell death, which is divided into two pathways, intrinsic
and extrinsic. Both pathways result in the activation of a group of caspases
and proteases that are responsible for cell death. In addition, these pathways
regulate apoptosis through proteins such as the B-cell lymphoma 2 (BCL-2)
family (33). Apigenin plays an important role
in apoptosis and its administration reduces cell survival. The function of
apigenin is intensified by the reduction of BCL-2 and B-cell lymphoma-extra
Large (BCL-XL) as well as the increase of
Bcl-2-associated X (BAX) protein (34, 35). Studies have indicated apigenin
causes apoptosis and cell growth inhibition in various tumors, including lung,
skin, blood, liver, breast, stomach, colon, and prostate, by modulating
different signaling pathways (36). Apigenin activates both intrinsic
and extrinsic pathways of apoptosis. In general, in the process of internal
pathway regulation, the mitochondrial membrane potential changes and leads to
the secretion of cytochrome C in the cytoplasm, which activates caspase 3 with
the formation of apoptotic protease activating factor (APAF), and as a result,
apoptosis occurs (37). And also, apigenin regulates the
extrinsic pathways of apoptosis by increasing the expression of mRNA of TNF-![]() , caspase-3,
and caspase 8 (36-38). In cancer
cells, apigenin induces apoptosis by regulating the expression of Bax, Bcl-2,
Akt, and signal transducer and activator of transcription 3 (STAT-3) proteins (37, 38).
, caspase-3,
and caspase 8 (36-38). In cancer
cells, apigenin induces apoptosis by regulating the expression of Bax, Bcl-2,
Akt, and signal transducer and activator of transcription 3 (STAT-3) proteins (37, 38).
The effect of apigenin on oxidative stress
Oxidative
stress is related to the imbalance between the antioxidant system and the
production of free radicals. In general, reactive oxygen species (ROS) are
essential in a limited amount for redox signaling and homeostasis of cells.
Excessive production of reactive oxygen species/ reactive nitrogen species
(ROS/RNS) neutralizes the body's defense system, which is called oxidative
stress. Oxidative stress can be related to cancer, cardiovascular diseases, eye
diseases, kidney disease, and diabetes. In addition, oxidative stress causes
oxidative changes including protein carbonylation, nitration, sulfoxidation,
lipid peroxidation, and DNA breaks such as single-strand breaks (SSB) and
double-strand breaks (DSB) (39, 40). Various
diseases, including cardiovascular diseases, diabetes, cancer, etc., are
related to excessive production of free oxygen species and oxidative stress.
Apigenin has significant antioxidant properties, such as enhancing enzymatic
and non-enzymatic antioxidants, free radical scavenging, and modulating
signaling pathways such as PI3/Akt, Nfr2, MAPK, and NF-KB. (41). Studies show that apigenin reduces
adhesion molecules expression, which can be a useful strategy against oxidative
stress, such as free-radical scavenging (42). Apigenin can also increase the
activity of antioxidant enzymes such as catalase (CAT), superoxide dismutase
(SOD), and glutathione synthetase (GSH-synthase) and also decreased the level
of malondialdehyde (MDA) to counteract oxidative
stress (43). In one of the studies to
investigate the antioxidant effect of apigenin, 25 mg/kg of apigenin was
administrated for two weeks. It was demonstrated that apigenin was able to
reduce the amount of lipid peroxidation product (malondialdehyde). On the other
hand, it increased the activities of antioxidant enzymes, including CAT, GPX,
and SOD as well as non-enzymatic antioxidants, such as vitamins C and E, which
led to a reduction in oxidative stress (52).
The effect of apigenin on cancer cells
The
anti-cancer property of apigenin is due to its ability to modulate various
signaling pathways including angiogenesis, apoptosis, tumor suppressor genes,
cell cycle, inflammation, and NF-κB, JAK/STAT3, PI3K/AKT/mTOR, MAPK/ER2,
Wnt/B-catenin pathways. Evidence shows that reactive oxygen species are of
great importance in the anti-tumor properties of apigenin (44). Apigenin can inhibit the invasion
and metabolism of cancer cells by regulating the production of protease (45). Studies indicate that apigenin
suppresses lung melanoma metastasis by eliminating the interaction of cancer
cells with the endothelium (46). Moreover,
the exposure of endothelial cells to apigenin can lead to the suppression of
vascular endothelial growth factors (VEGF) expression, which is an essential
factor in angiogenesis through the degradation of hypoxia-inducible factor 1-α (HIF-1a) protein (Figure 4) (47). Apigenin can also inhibit the expression of VEGF and HIF-1a
through human double minute 2 (HDM2)/P53 and PI3K/AKT/P70s6K1 pathways in
ovarian cancer cells (48).
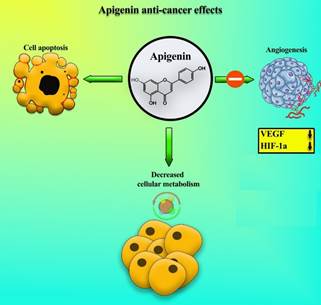
Figure 4. Anti-cancer
effects of apigenin, including inhibition of angiogenesis through vascular
endothelial growth factors (VEGF) suppression and protein hypoxia-inducible
factor 1-α (HIF-1a) degradation, reduction of metabolism, and activation of
apoptosis.
The
effect of apigenin on different cancers
Despite,
the significant progress made in cancer diagnosis and treatment in recent
years, it is still considered the second main cause of death in the world.
There are various modalities for cancer therapy, including hormone therapy,
radiation therapy, chemotherapy, and target therapy. Some of the main
challenges in cancer treatment, especially in the advanced stages, are the side
effects of drugs, chemical resistance, the killing of normal cells, and
treatment costs. Therefore, finding a treatment method with the least side
effects is very important and is in the preliminary stages. Using natural
products with strong therapeutic and preventive properties is of great value
and importance. It should be noted that their importance is because of reducing
the resistance of cancer cells to treatment and having fewer side effects (49, 50). Different studies have
demonstrated that high consumption of polyphenolic compounds such as flavonoids
can diminish the incidence of various cancers (51). In this study, we have tried to
show the effect of apigenin on several types of cancers.
Lung
cancer is one of the leading causes of death in the world (27). Biologically
and histologically, lung cancer is considered a complex neoplasm. The four main
histological kinds of lung cancer are small cell carcinoma, large cell
carcinoma, adenocarcinoma, and squamous cell carcinoma (62). Knekt et al (63),
investigated the relationship between the consumption of flavonoids such as
apigenin, quercetin, luteolin, and myricetin and lung cancer. They have found
that there is an inverse relationship between the occurrence of cancer and
flavonoid consumption. They have concluded that onion and apple, as two sources
rich in apigenin, can play a protective role against lung cancer. The
relationship between the consumption of flavonoids and their protective role in
the occurrence of various cancers, including breast cancer, ovarian cancer, and
colorectal cancer, has also been investigated (13, 52). Lui et al (64), have suggested
that apigenin could diminish the risk of lung cancer by inhibiting vascular
endothelial growth factor (VEGF) transcription and proliferation of A549 lung
cancer cells.
Gastric
cancer is one of the most common types of cancer around the world (53). There is compelling evidence that
Helicobacter pylori infection can be associated with gastric cancer. Therefore,
one of the preventive measures for gastric cancer is to eradicate the infection
of Heliobacter pylori (H.pylori). In addition, another strategy to reduce the
progress of gastric cancer is to use different flavonoid compounds such as
apigenin, which have significant antioxidant properties. In one of the
conducted studies, the effectiveness of apigenin on the progression of gastric
cancer and atrophic gastric caused by helicobacter pylori was investigated. And
the result showed that apigenin therapy significantly reduces the rates of
histological changes of neutrophils and monocyte infiltration as well as
H.pilori colonization in both gastric cancer and gastritis. In addition,
apigenin could dramatically increase the expression of IKBa. Therefore, it
could reduce the activation of NF-KB and inflammatory cytokines expression.
Moreover, the level of ROS diminished due to the scavenging characteristic of
apigenin (65, 66). Wu et al (67), evaluated the effect of apoptosis induction
and cell cycle inhibition of apigenin on SGC-7910 gastric carcinoma cells. They
observed that apigenin inhibits clone formation and growth of these cells
through apoptosis.
Neuroblastoma
causes approximately 15% of childhood cancer-related deaths (53). Neuroblastoma is one of the most
common extracranial solid tumors in children that originate from neural
progenitor cells. These tumors can occur in the central nervous system, pelvic
and thoracic regions. But they mainly appear in the abdominal region. Many
factors play a role in the occurrence of this disease, such as inflammation,
patient age, protein aggregation, tumor metastasis, etc. One of the important
risk factors of neuroblastoma is MYCN Proto-Oncogene amplification, which can
intensify neuroblastoma tumorigenesis. The age of the patient and elimination
of a protein from chromosome 11 (11q aberration) are other risk factors (54, 55). It's notable for the wide range of
clinical behavior. Some neuroblastoma tumors can differentiate into benign
types (benign ganglioneuromas) and some undergo sudden regression (56, 57). Therapeutic modalities for
neuroblastoma include surgery, chemotherapy, and radiotherapy (58). Stages 1 and 2 of the disease can
only be treated by surgery (59). But in higher stages, favorable
results are obtained with surgery and chemotherapy (60). Natural compounds have been proven
to have valuable anti-cancer properties. Some of these compounds with few side
effects can help prevent or even treat cancer. Flavonoids can suppress cancer
by epithelial-mesenchymal transition (EMT) inhibition, extracellular matrix
(ECM) protein modulation, and inhibiting the metabolism of cancer cells (61). Torkin et al (62), evaluated the effect of apigenin
on human neuroblastoma cell lines. They found that apigenin inhibits the
ability of colony formation and survival, and stimulates apoptosis in these
cell lines. Apigenin elevated p53 protein level and products derived from p53,
including Bax, p21WAF1/CIP1 gene. In addition, apigenin could increase the
activity of caspase-3 and cause cell death.
Thyroid cancer
and apigenin
Thyroid
cancer, as an unusual cancer, can account for about 1% of all malignancies (53). Thyroid cancer is known as the
fifth most prevalent cancer among women in the united states. The prevalence of
this cancer is rising around the world. Treatment modalities for thyroid cancer
in most patients are surgery combined with radioiodine therapy (63). Studies have shown that malignant
thyroid cancer is divided into different types, including follicular thyroid
cancer (FTC), papillary thyroid cancer (PTC), Hurthle cell cancer (HCC), and
anaplastic thyroid cancer (ATC), all of which are derived from epithelial
cancer cells. Other types include medullary thyroid cancer derived from
parafollicular and non-epithelial types such as teratoma, sarcoma, and
lymphoma. Among the different types of thyroid cancer, FTC, PTC, and HCC are
called differentiated thyroid cancer. While ATC is considered a very malignant
neoplasm. PTC is one of the most prevalent malignancies of thyroid cancer (64, 65). A study conducted on PCCL3 rat
thyroid cells showed that apigenin was able to increase iodide influx by
inhibiting AKT under thyrotropin stimulation (66). In addition, in the BCPAP cell
line, apigenin caused a considerable cell accumulation in the G2/M phase
through the reduction of cell division cycle 25 (Cdc25c) expression. Also,
apigenin suppressed the viability of PTC cells through the stimulation of ROS
production, which caused DNA damage and eventually resulted in autophagy cell
death (67). Yin et al (68), assessed the impact of some
flavonoid compounds such as apigenin on thyroid carcinoma cell lines, including
UCLA Ro-w-1(WRO) (follicular carcinoma), UCLA RO-81A-1(ARO) (anaplastic
carcinoma), and UClA NPA-87-1(NPA) (papillary carcinoma). Of all the flavonoids
used, apigenin has been the most effective proliferation inhibitor of cell
lines. Yin et al (69), in another study, showed that the
inhibitory impact of apigenin on the proliferation of ARO cells was related to
both phosphorylation of down-stream effector (MAPK) and epidermal growth factor
receptor (EGFR) tyrosine autophosphorylation.
Elst
et al (70), investigated the efficiency of
flavonoid compounds on the growth of follicular cell lines and iodine
transport. It was found that apigenin could suppress the expression of
Sodium-iodide symporter (NIS) mRNA, and this finding can have useful
therapeutic consequences for the treatment of thyroid cancer.
Liver cancer and apigenin
Liver
cancer, more precisely hepatocellular carcinoma, can be considered as the
second main reason for cancer deaths and its prevalence is rising worldwide (71). This disease occurs more in men
than women, and it is also more common in West and Middle Africa, South and
East Asia, and Melanesia (72). The consumption of flavonoids can
be effective in preventing Hepatocellular carcinoma (73). Flavonoids such as apigenin
reduced the survival of hepatocellular carcinoma HePG2 cells and induced
apoptosis by diminishing the expression of low-density lipoprotein
receptor-related protein (LRP6) and S-phase kinase-associated protein-2 (SKP2).
Further studies on apigenin against liver cancer cells indicated that this
anticancer agent suppressed cell proliferation and increased cell death. In
addition, apigenin caused autophagy and apoptosis by inhibiting the
phosphatidylinositol-3-kinase (PI3K/Akt) and mechanistic target of rapamycin
(mTOR) pathways. It has been indicated that apigenin therapy caused G1 arrest
in HepG2 cells. Also, the cells that were exposed to apigenin experienced an
increase in the amount of cyclin D1 and a decrease in cyclin 4, which indicates
that the cell cycle can be stopped by regulating the expression of CD1 and CDK4
(74, 75). Yee et al (76), studied the inhibitory efficiency
of two flavonoid compounds named apigenin and luteolin on Hepatocellular
carcinoma HepG2 cells. The results showed that both of these flavonoids had an
effective role in inhibiting cell growth, which was caused by diminishing the
expression of CDK4 and cell cycle arrest by inducing P21 and p53, respectively.
Skin
cancer is one of the most prevalent types of cancer in the united kingdom (UK)
and the united states (US) (77). The two most common types of skin
cancer are melanoma and non-melanoma skin cancer. Most skin cancers are related
to non-melanoma and result from keratinized epithelial cells. These types of
cancers can be divided into squamous cell carcinoma (SCC) and basal cell
carcinoma (BCC). BCC is the most common form and progresses slowly. Melanoma
accounts for approximately 2% of malignancies and causes the most mortality (77). It has been shown that ultraviolet B (UVB) radiation is the main
cause of this disease. Various studies have indicated that apigenin can be remarkably
effective in preventing skin carcinogenesis caused by ultraviolet A/B in SKH-1
mice (78). Caltagirone et al (79), investigated the combined impacts
of apigenin and quercetin on suppressing the metastatic, invasiveness, and
melanoma growth potential. They showed that the administration of quercetin and
apigenin under in vivo conditions inhibited the metastatic potential of
melanoma lung tumors in a BL6-BL6 murine model. This effect can be due to
demolishing the interaction between malignant and endothelial cells.
In
addition to skin cancer, another common cancer in men is prostate cancer (53). This cancer is one of the
multifactorial diseases. Prostate cancer is the second most common cancer and
the fifth main cause of death in the world. The prevalence and mortality rate
of prostate cancer is related to factors such as age, and the highest prevalence
can be seen in older men. The most prevalent therapeutic modalities are
surgery, radiotherapy, and/ or chemotherapy. It should be mentioned that these
options are efficient in the early stages and become ineffective in the higher
stages. This cancer can be reduced by
increasing the consumption of fruits and vegetables as well as reducing the
consumption of fatty foods. (80, 81). Flavonoids can be well tolerated
by prostate cells, but it should be noted that these natural compounds act as
mutagens, inhibitors of key regulatory enzymes, or pro-oxidant molecules in
case of excessive consumption. Various types of polyphenols have been studied
to kill prostate cancer cells(82, 83). In one of the studies, 22Rv1, PCa,
and PC3 cells were exposed to different concentrations of apigenin (20 and
40µM) for 24 hours. The results indicated that the activity of histone
deacetylation (HDAC) was reduced compared to that obtained from the famous HDAC
inhibitor trichostatin A (TSA). Also, apigenin decreased the regulation of
HDAC1 and HDAC3 at both protein and mRNA levels along with the simultaneous
increase in H3 and H4 acetylation. As a result, this causes the DNA promoter to
have more access to transcription factors and also, increases synthesis of cell
cycle regulating protein p21/waf1 in prostate cancer cells. P21/waf1 can
control cell cycle progression through cyclin-dependent kinase 2 (CDK2)
inhibition (84). Prostate cancer cells showed
induction of apoptotic pathways and cell cycle arrest 24 hours after apigenin
administration. In one of the in-vivo studies performed on PC3 xenografts in
athymic nude mice, the antitumor effect of apigenin was investigated. Oral
administration of apigenin (20 and 50 mg/mouse/d) during eight weeks caused a
significant decrease in HDAC1 and HDAC3 protein expression, HDAC activity, and
also a decrease in tumor growth. Mice were exposed to apigenin, the expression
of P21/waf1 was higher than the control group, and the change in the amount of
bax/bcl2 led to apoptosis induction (84). Knowles et al (85), evaluated the effectiveness of
apigenin on prostate cancer PC3 cell proliferation, and it was demonstrated
that when these cells are exposed to apigenin, their growth rate is delayed.
Hessenauer et al (86), indicated the relationship between
the growth of prostate cancer cells and casein kinase 2 (CK2) activity. They
found that apigenin was able to suppress the activity of CK2 in both
hormone-refractory PC3 and hormone-sensitive lymph node carcinoma of the
prostate (LNCap), but only the latter underwent apoptosis. This result
indicates that high activity of CK2 is not necessary for the proliferation and
protection of PC3 cells against apoptosis. A summary of the effectiveness of
apigenin on the mentioned cancers is indicated in Table 2.