Mechanisms,
challenges, and future prospects of the oncolytic virotherapy: a comprehensive
review
Fizza Maryam 1*, Sana Gul 2
1 Department of Biological Sciences,
National University of Medical Sciences, Islamabad, Pakistan
2 Department of Medicine, Institution
affiliation, New Mexico University, USA
Corresponding Authors: Fizza Maryam
* Email: fizza.maryam272000@gmail.com
Abstract
Oncolytic viruses (OVs) are a promising cancer-fighting agent that has
gained widespread attention due to recent advances in virology and molecular
biology. These viruses selectively infect and multiply inside tumor cells,
causing them to rupture and release newly synthesized viruses that stimulate
the body's immune system to target the tumor cells. Clinical investigations
have shown that OVs can effectively eliminate cancer cells that are resistant
to traditional treatments, which is why over 100 clinical trials are currently
exploring the possibility of combining them with other therapies for better
efficacy. Although OVs have demonstrated enormous potential, their
effectiveness in treating solid tumors is still limited. Therefore, researchers
are continuously developing new viral families that can exclusively replicate
in tumor cells. Currently, T-VEC is the only FDA-approved oncolytic virus, but
with ongoing phase I-III clinical studies, more promising treatments are on the
horizon. Furthermore, this review article provides a comprehensive overview of
OVs, including their mechanism of action delivery routes, challenges in
oncolytic virotherapy, current developments, the efficacy of OVs when combined
with other cancer treatments, and prospects for future research.
Keywords: Cancer, Cancer therapy, Clinical trials, Oncolytic viruses,
Virotherapy
Introduction
Genetic
and epigenetic changes transform normal cells into abnormal ones, which results
in cancer. Increasing numbers of cancer cases and deaths make cancer the second
leading cause of death worldwide. A WHO study estimates that there will be a
60% increase in cancer cases worldwide in the next 20 years (1). Cancer has
been recognized as a serious threat to human health and welfare. Chemotherapy,
radiotherapy, and surgical procedures could improve the survival rate in cancer
patients, but many patients with advanced cancer do not have access to these
treatments due to their high costs, especially in low- and middle-income
countries (LMICs). Studies have shown that significant disparities can occur in
treatment and outcomes due to the financial burden associated with cancer
treatment (2). Additionally,
advanced tumors often create an immunosuppressive environment that reduces the
effectiveness of traditional therapies (3). In this
context, oncolytic virotherapy offers a novel and potentially more affordable
treatment modality by leveraging viruses that can specifically target and
destroy cancer cells while activating the immune system. The particular
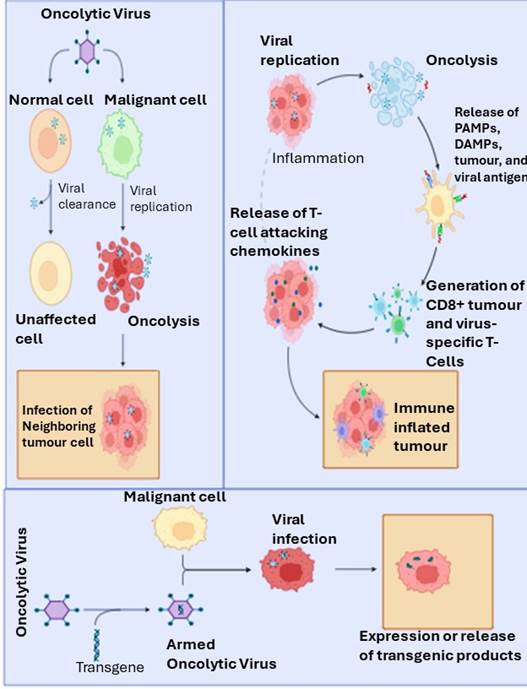
oncolytic viruses modulate immunological processes. These are viruses that
target specific types of cancer cells. They may be naturally occurring or
genetically modified. By incorporating a tumor-specific promoter element within
their genomes or by deleting essential portions of their genomes, so they
selectively replicate within cancer cells (4). In the 19th
century, researchers observed that viruses could selectively lyse cancer cells
rather than healthy cells, which led to the development of oncolytic virology,
the study of viruses that kill cancer cells. Various viral families have been
examined to use them as an oncolytic agent; several viruses have been in
preclinical studies during the past decade, and some have already been tested
in clinical trials (5). It's truly
remarkable how much progress has been made in the field of oncolytic
virotherapy. The advances in viral retargeting, viral delivery systems, gene
editing, tracking strategies, OV-based gene therapy, and combination approaches
have all contributed to expanding the potential applications of this therapy in
oncology. The possibilities for using these cutting-edge technologies to treat
and even cure cancer are truly exciting to consider. However, due to the
challenges associated with genetic engineering and safety concerns, oncolytic
virology has made little progress over the previous 20 years (4). The review
likely incorporates recent breakthroughs in virology and molecular biology that
have contributed to the understanding and development of oncolytic viruses.
This could include advancements in viral retargeting, and viral delivery
systems. Given the dynamic nature of research in this field, there may have
been discoveries of new viral families or innovative therapeutic approaches for
oncolytic virotherapy. The review likely discusses any new viruses that have
shown promise as oncolytic agents or novel strategies for enhancing the
efficacy of existing viruses. Overall, the review aims to highlight the
evolving landscape of oncolytic virotherapy and its potential in addressing the
challenges posed by cancer, showcasing the progress made in the field over the
past years and outlining avenues for future research.
History
Long
before the first official clinical trial using an OV was published in 1949,
several cases reported from the mid-1800s revealed that spontaneous microbial
infections could sometimes occasionally regress tumour burden in cancer
patients (6). A leukemic
patient in the late 1890s developed a "flu-like" illness that was
accompanied by generalized inflammation and a reduction in tumour cells,
providing additional proof of the therapeutic potential of viruses. In 1949,
the results of these studies led to the launch of several clinical trials at
Memorial Sloan-Kettering, treating more than 150 patients with wild-type RNA
viruses Bunyamwera (bunyaviridae), Ilheus (flaviviridae), Semliki Forest
(togaviridae), Newcastle disease (paramyxoviridae) West Nile (flaviviridae),
and Dengue (flaviviridae) (7, 8). In addition,
RIGVIR and Oncorine have received approval for use as OVs in various nations as
cancer treatments. In 2004, the Latvian government legalized the use of the
non-genetically virus strain RIGVIR, also known as enteric cytopathic human
orphan type 7, to cure melanoma (9, 10). In November
2005, the Chinese Food and Drug Administration approved the use of genetically
altered oncolytic adenovirus, known as H101 (Oncorine), in combination with
chemotherapy to treat nasopharyngeal cancer (11, 12). The oncolytic
virus T-VEC (Imlygic) also known as OncoVEXGM-CSF, a modified
version of the HSV-1, had been approved by the FDA
in 2015 to treat melanoma (13, 14). The deletion
of particular genes in the virus increases antigen presentation and promotes
selective replication within cancer cells (15). The approval
of T-VEC in 2015 gained the attention of researchers to work further on
oncolytic virotherapy to make them a powerful weapon against cancer in the
future.
Candidates
for the oncolytic virus
Currently,
extensive research suggests that DNA and RNA viruses, HSV, measles virus, and
many other viruses mentioned in (Table 1), are major candidates for cancer
therapy (16, 17). In
particular, adenoviruses and herpesviruses have been developed to precisely
detect and target cells expressing fetoprotein or prostate-specific antigen,
which is the cancer marker. Also, the surface proteins of the measles and
polioviruses were modified to alter their specificity to target only the
cancerous cells, not the healthy cells (18, 19).
Combination
of cancer treatment strategies with OV's
In
general, monotherapies alone are ineffective for treating cancer, especially in
metastatic or advanced stages. Certain types of cancer have already seen
significant improvements with the combination of numerous therapies. OVs are
using in combination with other anticancer treatments, such as immunotherapy,
drugs, and radiation. These can improve therapeutic outcomes, increase
therapeutic effectiveness, and focus on a larger variety of tumour types (64). Scientists
are just beginning to understand how oncolytic viruses work in conjunction with
chemotherapy and radiotherapy. A further benefit that makes OVs a desirably
combined platform is their engineering feasibility and confirmed safety
profiles (65). These OV-drug
combinations are clearly effective if they are chosen correctly, along with
properly chosen medications and the type of cancer attacked. Several
combination strategies have been tested for natural or synthetic OVs in recent
decades, both in the lab and in clinical trials. The majority of cancer
patients are still treated with chemotherapy. Combining chemotherapy with
oncolytic virotherapy causes a significant apoptotic induction in a number of
preclinical tumour models. For example, patients with advanced melanoma who
received T-VEC plus the immune checkpoint inhibitor ipilimumab showed improved
response rates compared to ipilimumab alone (66). Another study
demonstrated that patients with solid tumors who received an oncolytic
adenovirus along with pembrolizumab, another immune checkpoint inhibitor, had
durable responses (67). Likewise, Ad-H101was approved for the treatment of
cancer by China, particularly for the neck and head cancer in 2005 following
phase III clinical trials that revealed that, when Ad-H101 combined with chemotherapy with 5-FU which shows its effectiveness
upto 79-72 percent vs. 40 percent with chemotherapy alone (64, 68). Moreover, one
of the most prevalent cancer treatments is radiotherapy, which kills the cancer
cells, shrinks the tumour, and damages normal tissues and cells in the
surrounding area. When the human body is exposed to radiation, radionuclides
enter it. By promoting the accumulation of radionuclides in tumor cells, the
selective replicative capacity of OV can be enhanced to improve the precision
and safety of radiation therapies. The OVs can increase the susceptibility of
tumor cells to radiation, causing them to be more vulnerable to
radiation-induced damage (69). By disrupting
cellular repair mechanisms, viral infections enhance radiation treatment
effectiveness at lower doses, resulting in improved safety since healthy
tissues are not exposed to radiation (70). Additionally,
OVs can deliver radionuclides directly to tumor cells. It is possible to
deliver radiation to tumors specifically by engineering OVs to express or carry
radionuclide-conjugated proteins. In this way, radionuclide therapy is more
targeted, targeting cancer cells while limiting exposure to normal tissues.
Furthermore, combining OVs with radionuclide therapy could further broaden the
therapeutic window because of the dosage range within which the treatment is
effective and safe. The selective targeting and synergistic effects of OVs can
allow for lower doses of radiation to achieve the desired therapeutic effect,
improving overall safety and reducing side effects (66). Likewise,
there have been many studies conducted on the interaction between radionuclide
therapy and among those viruses that have been genetically modified to express
membrane protein, which is sodium iodide symporter
(NIS) that facilitates the cellular uptake of radionuclides such as 131I
(71-73). When vaccinia
viruses that express NIS are administrated prior to 131I treatment,
intramural production of NIS proteins raises the cellular content of
radioiodine, and the combined therapy is more effective in case of prostate
cancer cells as compare to use either OVs or 131I alone (74, 75). The results
of these studies suggest that OV-drug combinations can improve clinical
outcomes and enhance the immune response against tumors.
Delivery
of oncolytic viruses
When
conducting research, selecting the appropriate delivery method is crucial.
Researchers consider their research goals and the resources available to
determine the most effective approach. Oncolytic viruses are delivered to the
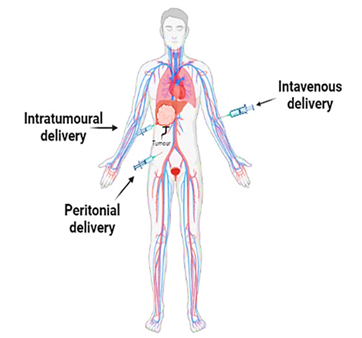
host via three routes: intravenous, intratumoral, and intraperitoneally.
Intrathecal and subcutaneous methods are utilized by researchers as
supplementary delivery routes, in addition to the three primary ones mentioned
above. There are some advantages and disadvantages of all these three routes.
In intravenous delivery, oncolytic viruses move throughout the circulatory
system when injected into a peripheral vein, reaching tumour lesions in
nonspecific organs and systems. It's an effective option when it is difficult
to directly introduce the oncolytic virus into tumour (76). In
preclinical and clinical settings, intravenous (IV) delivery are commonly used.
IV delivery allows the virus to circulate systemically and potentially reach
metastatic or deep-seated tumors. However, this method can face hurdles such as
the immune system neutralizing the virus before it reaches the tumor, and
limited virus penetration into the tumor microenvironment (77). The second
technique involves delivering oncolytic viruses directly to tumors for
treatment. This approach, known as intratumoral delivery, has a direct
therapeutic effect on the malignancy. This method delivers a concentrated dose
of oncolytic virus in vitro directly to the targeted tissue, allowing for a
clear and significant impact to be observed (78, 79).However, its application in vivo, particularly in deep
or inaccessible lesions, is challenging. Additionally, intraperitoneal
injection of the oncolytic virus into the peritoneum is the third delivery
route. Once absorbed, it either diffuses directly into tumour lesions within
the peritoneal cavity or into the peritoneum veins, where it reaches tumour
lesions via the circulatory system. The main benefit of this approach it's
simple to administer and requires few specialization skills. Compared to
subcutaneous injections, intraperitoneal injections are quickly absorbed (80). The
intraperitoneal is the best choice for treating abdominal organs, but it is
slowly absorbed compared to intravenous injection (Figure 3) (81, 82).
Figure
3.
Main delivery route of oncolytic viruses.
Challenges
and their solutions in oncolytic virotherapy
Even
though oncolytic virotherapy has great potential, it still faces many
challenges that need to be addressed for it to be more effective and safe.
There are several types of challenges that can be categorized as follows:
Immune-related
challenges
There
are many challenges and drawbacks of cancer-specific oncolytic virotherapy,
which include antiviral immune responses, antibodies frequently inactivating
circulating viruses, off-target infection, adverse conditions in the tumor
microenvironment, insufficient immunogenicity, and a number of barriers
inhibiting systemic delivery of oncolytic viruses (44, 83, 84). Host defense
system prevent the majority of oncolytic viruses from infecting tumours
following systemic delivery. When
delivering oncolytic viruses to the body, there are several obstacles that must
be overcome. These include blood cells, neutralizing antibodies, antiviral
cytokines, nonspecific uptake by other tissues, tissue-resident macrophages,
and difficulty in virus escape from the vascular compartment (85-87). This
technique needs a virus that preferentially infects tumour cells while remain
in the circulation without depleting or degrading.
Safety
concerns
Oncolytic
viruses have the potential to cause extensive organ damage and inflammation
when a significant volume of them are circulated throughout the body. For
replication-competent viruses, it may even be risky to assume that a few
mutations will modify these profiles entirely for scientific and clinical
purposes, but still it could be dangerous. For this reason, to assure safety,
preclinical assessments are necessary. Due to these restrictions, there may be
some discrepancies in the efficacy and safety margins between research on
animals and humans (88). The effects
of animal-derived oncolytic viruses can be studied in a variety of methods, but
it cannot be expected that the results gained in animal models would be
reproducible in people (84). The use of
oncolytic virotherapy may worsen comorbid conditions such as coagulopathies,
heart disease, liver disease, and lung disease (18). Antiviral
medication may already being administered to some individuals with chronic
viral infections, which could prevent viral oncolysis. Early clinical trials
revealed a phenomenon known as pseudoprogression, in which the treated tumours
grew larger and displayed more heterogeneity, likely as a result of infections
that caused inflammation or edema (89). Careful
consideration will also be given to the choice of patients. There is a
possibility that immunocompromised patients will not be suitable candidates due
to their weakened oncolytic virus-mediated antitumor immunity.
Research
and development challenges
Numerous
problems have arisen and will continue to arise in the field of virotherapy and
oncolytic research. Despite this, some efforts have been made to avoid or at
least mitigate the worst effects of viral infections and to improve the
effectiveness, safety, and usefulness of virotherapy (90). Making an
accurate diagnosis may require the development of novel molecular markers. For
example, human telomerase reverse transcriptase exhibits elevated expression
level in tumour cells but not in normal cells, which increases the
effectiveness of telomerase in targeting tumours and modifies the tropism of
viruses, allowing them to bind only to specific receptors on tumour cells, such
as the adenovirus Delta-24RGD (39, 91).
Delivery
challenges
The
main difficulty with this therapy is properly delivering the virus to the
tumour. Systemic administration rarely works because of preexisting immunity.
An off-target infection may occur where oncolytic viruses infect healthy cells
instead of tumor cells, causing unwanted side effects. As an example, a virus
that targets cancer cells in the liver might also infect healthy liver cells or
other tissues if it lacks sufficient specificity. A patient may experience
adverse effects due to this off-target infection causing damage to healthy
organs and tissues. A key to minimizing these risks is ensuring that the virus
only infects tumor cells and not healthy tissues. Since intratumoral injection
is costly and challenging, especially in cases of malignant gliomas, it is
necessary to optimize virus delivery in order to improve systemic delivery. The
use of complex viral particle ligands, nanoparticles, and immunomodulatory
drugs are a few of the novel strategies being studied (92). The
technically challenging image-guided delivery approach is used to introduce
viruses into tumours using nanoparticles.
Conclusion
and future prospect
OVs
have become promising immunotherapeutic treatments for advanced malignancies
over the past 20 years. Interest in oncolytic virotherapy increased after the US-FDA approved T-VEC in 2015. Several viruses have
been evaluated as prospective candidates for oncolytic virotherapy, including
vaccinia, reovirus, parvovirus, and picornavirus. Oncolytic virotherapy has not
become a common practice in medicine due to a number of biological and
technical obstacles. However, there are numerous OVs being tested in clinical
trials right now, and several aspects, such as the optimum way to administer
and their optimal combinations, are still being taken into consideration.
Several natural and genetically modified oncolytic viruses are now being
evaluated for monotherapy or combination therapy, and the majority of them seem
safe and have few dose-limiting toxicities. Some of these viruses have
progressed to various phases of clinical trials despite being in preclinical
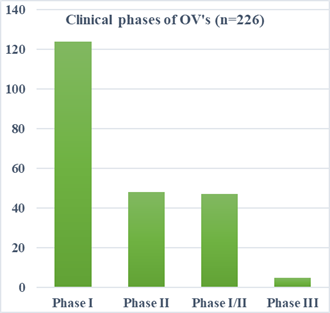
stages. A total of 153 trials are currently underway for DNA viruses and 70
trials for RNA viruses. As of 2021, 124 clinical studies indexed in PubMed were
Phase I trials, 47 Phase I/II trials, 48 Phase II trials, and five Phase III
trials. There have been numerous clinical
trials demonstrating the effectiveness of OVs in reducing tumor size and
improving patient survival rates, particularly when combined with other
treatment options. Oncolytic virotherapy could have a profound impact on cancer
treatment. In the future, new genetically altered OVs, new delivery techniques,
and new combination therapies will be developed. OVs will be the most effective
therapeutic approach for treating cancer once they have overcome the existing
obstacles to oncolytic virotherapy, such as physical obstacles, immunosuppressive TME, and host clearing of OVs. If
the issues mentioned above are properly resolved, oncolytic viruses could one
day be a perfect and painless therapeutic choice for cancer patients. To
effectively utilize OVs for novel approaches and overcome existing challenges,
a collaboration between the fields of immunology, molecular biology, structural
biology, genomics, and bioinformatics is necessary. In the near future,
oncolytic viral therapies should be developed further due to their continued
clinical need.
Author
contribution
Both
authors participated in the study design. The data collection was done by FM
and SG. The manuscript was written, reviewed, and edited by FM
and SG. Both authors read and
verified the final manuscript.
Conflict
of interest
The
authors have no conflict of interest.
Funding
The
authors express that no funds or other support were received during the
preparation of this manuscript.
References
1. Bray F, et al. Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 2024;74(3):229-63.
2. Mariotto AB, et al. Projections of the cost
of cancer care in the United States: 2010-2020. J Natl Cancer Inst.
2011;103(2):117-28.
3. Bates JP, et al. Mechanisms of immune
evasion in breast cancer. BMC Cancer. 2018;18(1):1-14.
4. Kaufman HL, Kohlhapp FJ. Oncolytic viruses:
a new class of immunotherapy drugs. Nature reviews Drug discovery.
2015;14(9):642-62.
5. Chaurasiya S, et al. Oncolytic virotherapy
for cancer: clinical experience. 2021;9(4):419.
6. Rahman MM, McFadden G. Oncolytic Viruses:
Newest Frontier for Cancer Immunotherapy. Cancers (Basel). 2021;13(21).
7. Zhang S, Rabkin SD. The discovery and
development of oncolytic viruses: are they the future of cancer immunotherapy?
Expert Opin Drug Discov. 2021;16(4):391-410.
8. Southam CM. Present status of oncolytic
virus studies. Trans N Y Acad Sci. 1960;22:657-73.
9. Alberts P, et al. Long-term treatment with
the oncolytic ECHO-7 virus Rigvir of a melanoma stage IV M1c patient, a small
cell lung cancer stage IIIA patient, and a histiocytic sarcoma stage IV
patient-three case reports. APMIS. 2016;124(10):896-904.
10. Doniņa S, et al. Adapted ECHO-7 virus Rigvir
immunotherapy (oncolytic virotherapy) prolongs survival in melanoma patients
after surgical excision of the tumour in a retrospective study. Melanoma Res.
2015;25(5):421-6.
11. Cao G-d, et al. The oncolytic virus in cancer
diagnosis and treatment. 2020;10:1786.
12. Zhang QN, et al. Recombinant human adenovirus
type 5 (Oncorine) reverses resistance to immune checkpoint inhibitor in a
patient with recurrent non-small cell lung cancer: A case report. Thorac
Cancer. 2021;12(10):1617-9.
13. Ferrucci PF, et al. Talimogene Laherparepvec
(T-VEC): An Intralesional Cancer Immunotherapy for Advanced Melanoma. Cancers
(Basel). 2021;13(6).
14. Pol J, et al. First oncolytic virus approved
for melanoma immunotherapy. Oncoimmunology. 2016;5(1):e1115641.
15. Howells A, et al. Oncolytic
Viruses-Interaction of Virus and Tumor Cells in the Battle to Eliminate Cancer.
Front Oncol. 2017;7:195.
16. Johnson PA, et al. Advances in DNA- and
RNA-Based Oncolytic Viral Therapeutics and Immunotherapies. 2022;2(2):319-29.
17. Watanabe D, Goshima F. c. Adv Exp Med Biol.
2018;1045:63-84.
18. Sze DY, et al. Oncolytic virotherapy. J Vasc
Interv Radiol. 2013;24(8):1115-22.
19. Dorer DE, Nettelbeck DM. Targeting cancer by
transcriptional control in cancer gene therapy and viral oncolysis. Advanced
Drug Delivery Reviews. 2009;61(7):554-71.
20. Rowan K. Oncolytic Viruses Move Forward in
Clinical Trials. JNCI: Journal of the National Cancer Institute.
2010;102(9):590-5.
21. Kaufman HL, Bines SD. OPTIM trial: a Phase
III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage
III or IV melanoma. Future Oncol. 2010;6(6):941-9.
22. Li H, et al. Coadministration of a herpes
simplex virus-2 based oncolytic virus and cyclophosphamide produces a
synergistic antitumor effect and enhances tumor-specific immune responses.
Cancer Res. 2007;67(16):7850-5.
23. Li H, et al. Virotherapy with a type 2 herpes
simplex virus-derived oncolytic virus induces potent antitumor immunity against
neuroblastoma. Clin Cancer Res. 2007;13(1):316-22.
24. Rodrigues R, et al. Bovine herpesvirus type 1
as a novel oncolytic virus. Cancer Gene Ther. 2010;17(5):344-55.
25. Hemminki O, et al. Oncolytic adenovirus based
on serotype 3. Cancer Gene Ther. 2011;18(4):288-96.
26. Wollmann G, et al. Targeting human
glioblastoma cells: comparison of nine viruses with oncolytic potential. J
Virol. 2005;79(10):6005-22.
27. Roos FC, et al. Oncolytic targeting of renal
cell carcinoma via encephalomyocarditis virus. EMBO Mol Med. 2010;2(7):275-88.
28. Adachi M, et al. Destruction of human
retinoblastoma after treatment by the E variantof encephalomyocarditis virus. J
Neurooncol. 2006;77(3):233-40.
29. Berry LJ, et al. Potent oncolytic activity of
human enteroviruses against human prostate cancer. Prostate. 2008;68(6):577-87.
30. Shafren DR, et al. Oncolysis of human ovarian
cancers by echovirus type 1. Int J Cancer. 2005;115(2):320-8.
31. Au GG, et al. Oncolysis of malignant human
melanoma tumors by Coxsackieviruses A13, A15 and A18. Virol J. 2011;8:22.
32. Toyoda H, et al. Oncolytic poliovirus therapy
and immunization with poliovirus-infected cell lysate induces potent antitumor
immunity against neuroblastoma in vivo. Int J Oncol. 2011;38(1):81-7.
33. Sturlan S, et al. Endogenous expression of
proteases in colon cancer cells facilitate influenza A viruses mediated
oncolysis. Cancer Biol Ther. 2010;10(6):592-9.
34. Galanis E, et al. Phase I trial of
intraperitoneal administration of an oncolytic measles virus strain engineered
to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res.
2010;70(3):875-82.
35. Myers R, et al. Oncolytic activities of
approved mumps and measles vaccines for therapy of ovarian cancer. Cancer Gene
Ther. 2005;12(7):593-9.
36. Hu J, et al. Selective in vitro cytotoxic
effect of human cancer cells by bluetongue virus-10. Acta Oncol.
2008;47(1):124-34.
37. Pol J, et al. Oncolytic viruses: A step into
cancer immunotherapy. Virus Adaptation and Treatment. 2011;2012:4:1-21.
38. Hanahan D, Weinberg RA. Hallmarks of cancer:
the next generation. Cell. 2011;144(5):646-74.
39. Bai Y, et al. Updates to the antitumor
mechanism of oncolytic virus. Thorac Cancer. 2019;10(5):1031-5.
40. Filley AC, Dey M. Immune System, Friend or
Foe of Oncolytic Virotherapy? Front Oncol. 2017;7:106.
41. Otto T, Sicinski P. Cell cycle proteins as
promising targets in cancer therapy. Nat Rev Cancer. 2017;17(2):93-115.
42. Wang L, et al. Remodeling the tumor
microenvironment by oncolytic viruses: beyond oncolysis of tumor cells for
cancer treatment. Journal for immunotherapy of cancer. 2022;10(5):e004167.
43. Liu Y, Zeng G. Cancer and innate immune
system interactions: translational potentials for cancer immunotherapy. J
Immunother. 2012;35(4):299-308.
44. Goradel NH, et al. Oncolytic virotherapy:
Challenges and solutions. 2021;45(1):100639.
45. Tornesello AL, et al. Virus-like Particles as
Preventive and Therapeutic Cancer Vaccines. 2022;10(2):227.
46. Garmaroudi GA, et al. Therapeutic Efficacy of
Oncolytic Viruses in Fighting Cancer: Recent Advances and Perspective. Oxid Med
Cell Longev. 2022;2022:3142306.
47. Boagni DA, et al. Current strategies in
engaging oncolytic viruses with antitumor immunity. Molecular Therapy -
Oncolytics. 2021;22:98-113.
48. Tian Y, et al. Engineering strategies to
enhance oncolytic viruses in cancer immunotherapy. Signal Transduction and
Targeted Therapy. 2022;7(1):117.
49. Mashima H, et al. Generation of
GM-CSF-producing antigen-presenting cells that induce a cytotoxic T
cell-mediated antitumor response. Oncoimmunology. 2020;9(1):1814620.
50. de Graaf JF, et al. Armed oncolytic viruses:
A kick-start for anti-tumor immunity. Cytokine Growth Factor Rev.
2018;41:28-39.
51. Zhao Y, et al. Oncolytic Adenovirus:
Prospects for Cancer Immunotherapy. Front Microbiol. 2021;12:707290.
52. Jhawar SR, et al. Oncolytic Viruses-Natural
and Genetically Engineered Cancer Immunotherapies. Front Oncol. 2017;7:202.
53. Groeneveldt C, et al. Immunotherapeutic
Potential of TGF-β Inhibition and Oncolytic Viruses. Trends Immunol. 2020;41.
54. de la Nava D, et al. Immunovirotherapy for
Pediatric Solid Tumors: A Promising Treatment That is Becoming a Reality. Front
Immunol. 2022;13:866892.
55. ClinicalTrials.gov. U.S. National Library of
Medicine [Available from: https://clinicaltrials.gov.
56. Lauer UM, Beil J. Oncolytic viruses:
challenges and considerations in an evolving clinical landscape.
2022;18(24):2713-32.
57. Macedo N, et al. Clinical landscape of
oncolytic virus research in 2020. Journal for immunotherapy of cancer.
2020;8(2).
58. Hemminki O, et al. Oncolytic viruses for
cancer immunotherapy. J Hematol Oncol. 2020;13(1):84.
59. Raman SS, et al. Talimogene laherparepvec:
review of its mechanism of action and clinical efficacy and safety.
Immunotherapy. 2019;11(8):705-23.
60. Sugawara K, et al. Oncolytic herpes virus
G47Δ works synergistically with CTLA-4 inhibition via dynamic intratumoral
immune modulation. Mol Ther Oncolytics. 2021;22:129-42.
61. Zeng J, et al. Oncolytic Viro-Immunotherapy:
An Emerging Option in the Treatment of Gliomas. Front Immunol. 2021;12:721830.
62. Mondal M, et al. Recent advances of oncolytic
virus in cancer therapy. Hum Vaccin Immunother. 2020;16(10):2389-402.
63. Yang L, et al. Oncolytic Virotherapy: From
Bench to Bedside. Front Cell Dev Biol. 2021;9:790150.
64. Wennier S, et al. Bugs and Drugs: Oncolytic
Virotherapy in Combination with Chemotherapy. Curr Pharm Biotechnol.
2011;13:1817-33.
65. Engeland CE, et al. Improving
immunovirotherapies: the intersection of mathematical modelling and
experiments. ImmunoInformatics. 2022;6:100011.
66. Chesney J, et al. Randomized, Open-Label
Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec
in Combination With Ipilimumab Versus Ipilimumab Alone in Patients With
Advanced, Unresectable Melanoma. J Clin Oncol. 2018;36(17):1658-67.
67. Andtbacka RH, et al. Talimogene Laherparepvec
Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin
Oncol. 2015;33(25):2780-8.
68. Garber K. China Approves World's First
Oncolytic Virus Therapy For Cancer Treatment. JNCI: Journal of the National
Cancer Institute. 2006;98(5):298-300.
69. Peter M, Kühnel F. Oncolytic Adenovirus in
Cancer Immunotherapy. Cancers (Basel). 2020;12(11).
70. Gujar SA, et al. Oncolytic virus-initiated
protective immunity against prostate cancer. Mol Ther. 2011;19(4):797-804.
71. Goel A, et al. Radioiodide imaging and
radiovirotherapy of multiple myeloma using VSV(Delta51)-NIS, an attenuated
vesicular stomatitis virus encoding the sodium iodide symporter gene. Blood.
2007;110(7):2342-50.
72. Opyrchal M, et al. Effective radiovirotherapy
for malignant gliomas by using oncolytic measles virus strains encoding the
sodium iodide symporter (MV-NIS). Hum Gene Ther. 2012;23(4):419-27.
73. Galanis E, et al. Oncolytic measles virus
expressing the sodium iodide symporter to treat drug-resistant ovarian cancer.
Cancer Res. 2015;75(1):22-30.
74. Zhang B, Cheng P. Improving antitumor
efficacy via combinatorial regimens of oncolytic virotherapy. Mol Cancer.
2020;19(1):158.
75. Mansfield DC, et al. Oncolytic vaccinia virus
as a vector for therapeutic sodium iodide symporter gene therapy in prostate
cancer. Gene Ther. 2016;23(4):357-68.
76. Hu C, et al. Intravenous injections of the
oncolytic virus M1 as a novel therapy for muscle-invasive bladder cancer. Cell
Death Dis. 2018;9(3):274.
77. Bauzon M, Hermiston TW. Oncolytic viruses:
the power of directed evolution. Adv Virol. 2012;2012:586389.
78. Fend L, et al. Immune Checkpoint Blockade,
Immunogenic Chemotherapy or IFN-α Blockade Boost the Local and Abscopal Effects
of Oncolytic Virotherapy. Cancer Res. 2017;77(15):4146-57.
79. Selman M, et al. Dimethyl fumarate
potentiates oncolytic virotherapy through NF-κB inhibition. Sci Transl Med.
2018;10(425).
80. Chen CY, et al. Cooperation of Oncolytic
Herpes Virotherapy and PD-1 Blockade in Murine Rhabdomyosarcoma Models. Sci
Rep. 2017;7(1):2396.
81. Li L, et al. Delivery and Biosafety of
Oncolytic Virotherapy. Front Oncol. 2020;10:475.
82. O'Leary MP, et al. Novel oncolytic chimeric
orthopoxvirus causes regression of pancreatic cancer xenografts and exhibits
abscopal effect at a single low dose. J Transl Med. 2018;16(1):110.
83. Yang M, et al. Cancer Immunotherapy and
Delivery System: An Update. 2022;14(8):1630.
84. Davis JJ, Fang B. Oncolytic virotherapy for
cancer treatment: challenges and solutions. J Gene Med. 2005;7(11):1380-9.
85. Ferguson MS, et al. Systemic delivery of
oncolytic viruses: hopes and hurdles. Adv Virol. 2012;2012:805629.
86. Wong HH, et al. Oncolytic Viruses for Cancer
Therapy: Overcoming the Obstacles. Viruses. 2010;2(1):78-106.
87. Shashkova EV, et al. Macrophage depletion
combined with anticoagulant therapy increases therapeutic window of systemic
treatment with oncolytic adenovirus. Cancer Res. 2008;68(14):5896-904.
88. Hamidi-Sofiani V, et al. Oncolytic viruses
and pancreatic cancer. Cancer Treatment and Research Communications.
2022;31:100563.
89. Sze DY, et al. Dr. Gary J. Becker Young
Investigator Award: Intraarterial Adenovirus for Metastatic Gastrointestinal
Cancer: Activity, Radiographic Response, and Survival. J Vasc Interv Radiol.
2003;14(3):279-90.
90. Chaurasiya S, Fong Y. Viroimmunotherapy for
breast cancer: promises, problems and future directions. Cancer Gene Ther.
2021;28(7):757-68.
91. Fujiwara T. Multidisciplinary oncolytic
virotherapy for gastrointestinal cancer. 2019;3(4):396-404.
92. Yokoda R, et al. Oncolytic virus delivery:
from nano-pharmacodynamics to enhanced oncolytic effect. Oncolytic Virother.
2017;6:39-49.