Figure
1. Correlation
Analysis Between AFP and Liver Function Tests.
Figure
2. Quarterly
Variations of Alpha Fetoprotein Levels in HBV/HCV Co-infection and HBV Monoinfection Groups among Subjects in Warri.
Alpha-fetoprotein
as a predictor of liver disease progression in HBV patients with HIV and HCV
co-infections
Mathew Folaranmi Olaniyan 1,
Kemi Felicia Ajiboye 2, Ogbeche Richard Ochagu 3, Tolulope Busayo Olaniyan 1,4,
Taiwo Medinat Adeniran 4, Adelani Wakili Tijani 5, Phoebe
Nwamaka Kanikwu 6, Obataze
Josephine Akpoyovwere 6, Odekunle Bola Odegbemi
1,7 *
1 Medical Laboratory Science Department, Edo
State University, Uzairue, Edo State, Nigeria
2 Public Health Department, Torrens
University, Adelaide, Australia
3 Directorate of Medical Services, Nigerian
Navy Headquarters, Abuja, Nigeria
4 Oyo State College of Nursing, Eleyele, Ibadan, Oyo State, Nigeria
5 Nursing Science Department, Federal
University Oye Ekiti, Ekiti State Nigeria
6 Nursing Science Department, Edo State
University, Uzairue, Edo State, Nigeria
7 Medical Laboratory Science Department,
Nigerian Navy Hospital, Warri, Delta State, Nigeria
Corresponding Authors: Odekunle Bola Odegbemi
* Email: odegbemi21.odekunle@edouniversity.edu.ng
Abstract
Introduction: Hepatitis B virus
(HBV) infection is a significant health challenge globally, especially in
sub-Saharan Africa. Co-infections with HIV and HCV worsen HBV-related liver
diseases, complicating clinical management. Alpha-fetoprotein (AFP) is a key
biomarker for monitoring liver disease progression and detecting hepatocellular
carcinoma (HCC). This study evaluates AFP levels in HBsAg and HBeAg seropositive patients with and without HIV and HCV
co-infections over one year in Warri, Delta State, Nigeria. This study aimed to
understand the impact of HIV and HCV co-infections on liver disease prognosis
in HBV patients by evaluating AFP levels and liver function over one year.
Materials and Methods: This longitudinal cohort study included 200 HBsAg and HBeAg seropositive patients aged 18-65 years, divided into
three groups: HBV monoinfection (n=80), HBV/HIV
co-infection (n=60), and HBV/HCV co-infection (n=60). Participants were
followed for one year with quarterly blood sample collections for AFP
measurement using ELISA, liver function tests (ALT, AST, ALP, bilirubin), and
viral load assessments. Sociodemographic data were also collected.
Results: AFP levels were significantly higher in the HBV/HCV co-infection
group (36.2 ± 12.4 ng/mL) compared to the HBV monoinfection
(12.5 ± 4.3 ng/mL) and HBV/HIV co-infection groups (18.7 ± 6.8 ng/mL)
(p<0.001). Elevated liver function tests, particularly ALT and AST, were
more prevalent in the HBV/HCV co-infection group. AFP levels positively
correlated with ALT (r=0.52, p<0.01) and AST (r=0.47, p<0.01) in the
HBV/HCV co-infection group.
Conclusion: The higher AFP levels in HBV/HCV co-infected patients indicate an
increased risk of liver disease progression and HCC. The positive correlations
between AFP and liver enzymes suggest ongoing liver damage and regeneration in
this group. These findings underscore the importance of routine AFP and liver
function tests in the early detection and treatment of liver disease among HBV
patients, particularly those with HCV co-infection, to enhance clinical
outcomes.
Keywords: Alpha-fetoprotein (AFP), Hepatitis B virus (HBV), Hepatitis C virus
(HCV), Liver function tests, Hepatocellular carcinoma, Co-infection
Introduction
Hepatitis
B virus (HBV) infection continues to be a significant global health challenge,
particularly in sub-Saharan Africa, where the prevalence remains high (1, 2).
Complicating the clinical landscape, co-infections with human immunodeficiency
virus (HIV) and hepatitis C virus (HCV) are common, exacerbating the
progression of liver diseases associated with HBV. Co-infections pose
additional challenges in clinical management and prognosis, necessitating more
in-depth studies to understand their interactions and effects on liver health
(1, 2).
Alpha-fetoprotein
(AFP) serves as a crucial biomarker in monitoring liver disease progression and
detecting hepatocellular carcinoma (HCC). Elevated AFP levels are often
associated with liver inflammation, regeneration, and malignancy (3). Despite
its widespread use, the dynamics of AFP levels in HBV patients with concurrent
HIV and HCV infections remain inadequately explored(4).
This study focuses on evaluating AFP levels in HBsAg and HBeAg
seropositive patients, both with and without HIV and HCV co-infections, over
one year in Warri, Delta State, Nigeria. The findings aim to provide insights
into the impact of these viral interactions on liver disease prognosis and AFP
variability (3–6).
In-depth
knowledge of the implications of anti-HBe in HBV
infection is crucial for comprehensive disease management. The presence of
hepatitis B e-antigen (HBeAg) in the blood typically
indicates active viral replication and high infectivity (7). Conversely, the
appearance of antibodies against HBeAg (anti-HBe) usually suggests a transition to a lower replicative
state of the virus, which is often associated with a more favorable prognosis
(8–11). However, this seroconversion does not necessarily mean that the virus
has been cleared from the liver. It signifies that the immune system has
responded to the virus in a way that reduces its replication (7–11).
Liver
disease in HBV patients, particularly in those with co-infections, poses a
complex challenge for clinical management (12–14). Co-infection with HIV and
HCV can alter the natural course of HBV infection, leading to more severe liver
damage and an increased risk of HCC. HIV co-infection, for instance, can
accelerate the progression of liver fibrosis and increase the likelihood of
cirrhosis and liver-related mortality. Similarly, HCV co-infection can result
in more aggressive liver disease and complicate treatment outcomes. Therefore,
scientific knowledge on how these co-infections influence AFP levels and liver
disease progression is vital for improving patient outcomes (12–14).
The
primary objective of this study is to assess the levels of AFP in HBsAg and HBeAg seropositive patients, with and without HIV and HCV
co-infections, over one year. This evaluation will help elucidate the influence
of co-infections on liver disease progression and the potential development of
HCC. By examining AFP levels longitudinally, this research aims to highlight
any significant fluctuations that could be indicative of disease progression or
response to therapy (15-17).
Materials and methods
Study
Design and Population
This longitudinal cohort study was conducted over
one year, involving 200 HBsAg and HBeAg seropositive
patients aged 18-65 years, recruited from healthcare facilities in Warri, Delta
State, Nigeria.
Sample
Size Determination
The sample size was calculated using the formula:
n = Z² * P(1-P) / d²
Where:
n = required sample size
Z = 1.96 (for 95% confidence level)
P = 0.109 (10.9% prevalence) (18)
d = 0.05 (5% precision)
n = (1.96)² *
0.109(1-0.109) / (0.05)²
n = 3.8416 * 0.109 * 0.891 / 0.0025
n = 149.82
Rounding up to the nearest whole number: 150
To account for potential non-response or dropout,
15% proportion was added to the minimum sample size obtained
150 + (150 * 0.15) = 172.5
Accordingly, a minimum sample size of 173 subjects
was appropriate for the study to achieve a 95% confidence level with 5%
precision. However, to improve diversity in study participation and to ensure
greater precision, 200 subjects were recruited for the study (19).
The study population was categorized into three
groups:
1.
HBV monoinfection without HBeAb
expression (n=80)
2.
HBV/HIV
co-infection without HBeAb expression (n=60)
3.
HBV/HCV
co-infection without HBeAb expression (n=60)
Inclusion
and Exclusion Criteria
Participants were included if they were seropositive
for HBsAg and HBeAg and had no expression of HBeAb. Exclusion criteria included prior liver disease,
HCC, or other significant co-morbidities.
Ethical
Considerations
This study obtained Ethical Approval from the Delta
State Ministry of Health Research and Ethics Review Committee. We adhered to
ethical principles, including:
i. Informed
Consent: Each participant received a written informed consent form alongside
the questionnaire, ensuring their consent to participate.
ii. Data Confidentiality: Findings from the study
were kept confidential and shared only among co-investigators.
iii. Beneficence: The results of the findings were
provided to the managing clinical team without any charge.
iv. Voluntariness: Both cases and controls had the
option to decline participation in the study when approached.
Data
Collection
Blood samples were collected quarterly for one year.
Alpha-fetoprotein (AFP) levels were measured using enzyme-linked immunosorbent
assay (ELISA). Additional tests included liver function tests (ALT, AST, ALP,
and bilirubin) and viral load assessments. Sociodemographic data, including
age, gender, occupation, and lifestyle factors, were collected using structured
questionnaires.
Principles
of Assays for Laboratory Analysis
Alpha-fetoprotein
(AFP) Measurement
AFP levels were determined using a commercial
Enzyme-Linked Immunosorbent Assay (ELISA) kit (Bio-Rad kit). The principle of
ELISA involves the following steps:
1.
Antigen-Antibody Binding: The AFP in the patient’s sample binds to the specific antibodies coated
on the wells of the ELISA plate.
2.
Washing: Unbound substances are removed through washing.
3.
Enzyme-Linked Secondary Antibody: An enzyme-linked secondary antibody specific to AFP is added, which
binds to the AFP already captured by the primary antibody.
4.
Substrate Addition: A substrate is added that the enzyme converts to a detectable signal,
typically a color change.
5.
Detection: The intensity of the color is measured using a spectrophotometer and is
proportional to the AFP concentration in the sample.
Liver
Function Tests
Liver function tests (LFTs) including ALT, AST, ALP,
and bilirubin levels were measured using automated biochemical analyzers (Biobase BS-230). The principles of these tests are as
follows:
1.
Alanine Aminotransferase (ALT) and
Aspartate Aminotransferase (AST):
o
Enzyme Activity Measurement: ALT and AST catalyze the transfer of amino groups from alanine and
aspartate to alpha-ketoglutarate, respectively. The reaction produces pyruvate
and oxaloacetate, which are then converted to a detectable product.
o
Spectrophotometry: The change in absorbance is measured, reflecting enzyme activity.
2.
Alkaline Phosphatase (ALP):
o
Enzyme Activity Measurement: ALP catalyzes the hydrolysis of phosphate esters, releasing inorganic
phosphate.
o
Spectrophotometry: The release of phosphate is measured, indicating enzyme activity.
3.
Bilirubin:
o
Direct and Total Bilirubin
Measurement: Bilirubin reacts with diazo reagent to
form azobilirubin, which is measured spectrophotometrically.
o
Indirect Bilirubin Calculation: Indirect bilirubin is calculated by subtracting direct bilirubin from
total bilirubin.
HBsAg,
HBeAg, Anti-HBe, Anti-HCV,
and HIVp24 detection
These markers were measured using ELISA kits
(Bio-Rad kit), and the principles are similar to the AFP ELISA described above:
1.
Antigen/Antibody Binding: The specific antigen or antibody in the patient's sample binds to the
corresponding antibody or antigen coated on the ELISA plate.
2.
Washing: Unbound components are washed away.
3.
Enzyme-Linked Secondary Antibody: An enzyme-linked secondary antibody specific to the target antigen or
antibody is added, binding to the antigen-antibody complex.
4.
Substrate Addition: A substrate is added that is converted by the enzyme into a detectable
signal.
5.
Detection: The resulting color change is measured, which is proportional to the
concentration of the target antigen or antibody in the sample.
Quality
Control Measures
ELISA
Assay: The ApDia ELISA
semi-autoanalyzer was used to AFP, HBsAg, HBeAg,
Anti-HBe, Anti-HCV, and HIVp24 detection levels in
plasma samples. Quality control measures were implemented to ensure the
accuracy and reliability of the assay results. External positive and negative
controls, provided by the manufacturer, were tested concurrently with each
batch of plasma samples. These controls were essential for verifying the proper
functioning of the test kits and ensuring that each assay run was valid.
Additionally, calibration curves were generated using standard solutions, and
the consistency of these curves was monitored across different assay batches.
Liver
Function Tests: Liver function tests were conducted by
spectrophotometric method using Biobase autoanalyzer
(BS-230), which was calibrated regularly to maintain precision. The tests
included measurements of ALT, AST, ALP, and bilirubin levels. Quality control
was a critical component of the testing process, with both internal and
external controls employed. The external controls, provided by the
manufacturer, were tested alongside the plasma samples to verify the accuracy
of the test kits and the reliability of the results. These controls were run
with every batch to confirm the proper performance of the analyzer.
Quality
Control Measures
To ensure the robustness of the data, stringent
quality control measures were implemented throughout the study. External
positive and negative controls were run concurrently with each assay to verify
the correct functioning of the analytical instruments and test kits.
Additionally, calibration and internal control procedures were rigorously
followed to minimize inter- and intra-assay variability.
Data
Cleaning
Before proceeding with data analysis, all collected
data underwent a thorough cleaning process. This step involved checking for any
inconsistencies, outliers, or missing values that could affect the accuracy of
the final results.
Statistical
Analysis
The cleaned data were then analyzed using SPSS
version 25. AFP levels were compared between groups using one-way ANOVA,
followed by post hoc Tukey tests for pairwise comparisons. Pearson's
correlation coefficient assessed correlations between AFP levels and clinical
parameters. Temporal variations in AFP levels were analyzed using repeated
measures ANOVA. A p-value <0.05 was considered statistically significant.
Results
The mean age of the participants was 42 ± 10 years,
with a male-to-female ratio of 1.2:1. No significant differences were observed
in age, gender, or socioeconomic status between the groups (Table 1).
Table
1. Sociodemographic
Characteristics of Study Participants.
|
Characteristic |
HBV Monoinfection (n=80) |
HBV/HIV Co-infection (n=60) |
HBV/HCV Co-infection (n=60) |
p-value |
|
Age (years) |
42.3 ± 9.8 |
41.7 ± 10.2 |
42.8 ± 9.5 |
0.87 |
|
Gender (M/F) |
44/36 |
32/28 |
33/27 |
0.72 |
|
Socioeconomic Status (Low/Medium/High) |
34/30/16 |
25/23/12 |
28/22/10 |
0.81 |
AFP
Levels and Liver Function Tests
AFP levels were significantly higher in the HBV/HCV
co-infection group (36.2 ± 12.4 ng/mL) compared to the HBV monoinfection
(12.5 ± 4.3 ng/mL) and HBV/HIV co-infection groups (18.7 ± 6.8 ng/mL)
(p<0.001) (Table 2). Elevated liver function tests, particularly ALT and
AST, were also more prevalent in the HBV/HCV co-infection group.
Table
2. AFP
Levels and Liver Function Tests in Study Groups.
|
Parameter |
HBV Monoinfection (n=80) |
HBV/HIV Co-infection (n=60) |
HBV/HCV Co-infection (n=60) |
p-value |
|
AFP (ng/mL) |
12.5 ± 4.3 |
18.7 ± 6.8 |
36.2 ± 12.4 |
<0.001 |
|
ALT (U/L) |
32.4 ± 10.2 |
45.7 ± 15.3 |
62.8 ± 20.1 |
<0.001 |
|
AST (U/L) |
28.3 ± 9.5 |
40.2 ± 12.8 |
59.4 ± 18.6 |
<0.001 |
|
ALP (U/L) |
110.7 ± 32.1 |
122.6 ± 38.4 |
135.2 ± 41.7 |
0.02 |
|
Bilirubin
(mg/dL) |
1.1 ± 0.3 |
1.4 ± 0.4 |
1.8 ± 0.6 |
<0.001 |
Correlation
Analysis
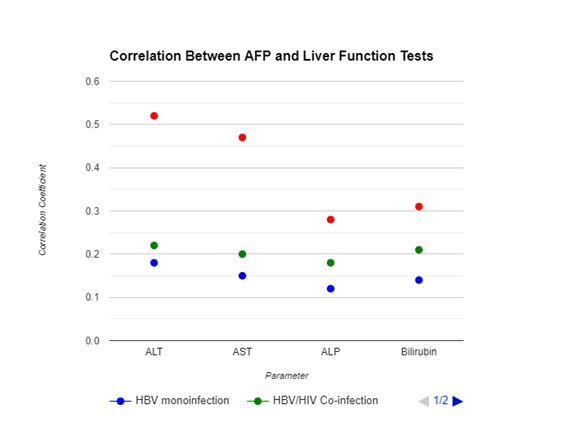
AFP levels positively correlated with ALT (r=0.52,
p<0.01) and AST (r=0.47, p<0.01) in the HBV/HCV co-infection group. No
significant correlations were observed in the HBV monoinfection
or HBV/HIV co-infection groups (Figure 1).
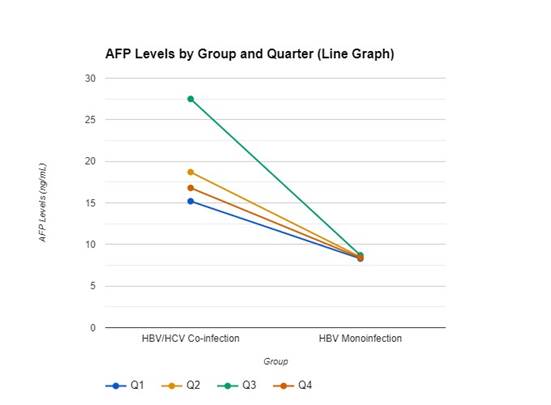
Figure 2 shows Alpha-Fetoprotein (AFP) levels
measured over four quarters, (Q1 to Q4) for two groups of patients: those with
both Hepatitis B and C (HBV/HCV co-infection) and those with only Hepatitis B
(HBV monoinfection).

Figure
1. Correlation
Analysis Between AFP and Liver Function Tests.

Figure
2. Quarterly
Variations of Alpha Fetoprotein Levels in HBV/HCV Co-infection and HBV Monoinfection Groups among Subjects in Warri.
Discussion
This
study evaluated Alpha-Fetoprotein (AFP) levels in HBsAg and HBeAg
seropositive patients with and without HIV and HCV co-infections in Warri,
Delta State, Nigeria, over one year. Our findings indicate that AFP levels are
significantly higher in patients with HBV/HCV co-infection compared to those
with HBV monoinfection or HBV/HIV co-infection. These
elevated AFP levels in the HBV/HCV group suggest a heightened risk of liver
disease progression and potential hepatocellular carcinoma (HCC) development.
This observation aligns with previous studies that identified HCV co-infection
as a factor that exacerbates liver disease in HBV patients (20-23).
The
elevated AFP levels observed in HBV/HCV co-infected patients compared to those
with HBV monoinfection or HBV/HIV co-infection
underscore the additive or synergistic hepatocellular damage inflicted by HCV.
HCV is known for its direct cytopathic effects and its ability to exacerbate
liver inflammation, leading to more significant liver injury and, consequently,
higher AFP levels. This is consistent with previous studies highlighting the
exacerbation of liver disease in the presence of HCV, which may explain the
pronounced increase in AFP levels observed in our study (24-27).
In
contrast, the relatively modest increase in AFP levels among HBV/HIV
co-infected patients suggests a different interaction between HIV and HBV in
the liver. Although HIV is associated with chronic immune activation and
inflammation, its direct impact on hepatocytes may be less pronounced compared
to HCV. The immunosuppressive nature of HIV may also modulate the inflammatory
response in a manner that does not significantly elevate AFP levels, despite
the ongoing liver damage (28-31).
It
is also possible that the antiretroviral therapy (ART) used in HIV-infected
individuals plays a role in mitigating liver injury and, by extension, AFP
production. ART has been shown to reduce HIV viral load and associated immune
activation, potentially attenuating the extent of liver damage and AFP
elevation in HBV/HIV co-infected individuals (23). However, the hepatotoxic
potential of certain ART drugs cannot be ignored, and further research is
needed to disentangle these complex interactions (24).
Another
factor to consider is the differential immune response elicited by HCV and HIV
in co-infected patients. HCV's ability to induce a more robust and sustained
inflammatory response in the liver, as opposed to the more systemic immune
dysregulation seen in HIV infection, might explain the observed differences in
AFP levels. HCV's propensity to cause chronic liver inflammation and fibrosis
may lead to increased AFP production as a marker of ongoing liver regeneration
and damage (32-38).
Moreover,
the role of AFP as a biomarker in these co-infection settings is multifaceted.
While elevated AFP is a well-known marker for hepatocellular carcinoma (HCC),
its utility in monitoring chronic liver disease progression, especially in
co-infected patients, remains an area of active investigation (39-41). The
correlation between AFP levels and liver function tests (LFTs) observed in this
study further supports its potential role in tracking liver disease severity,
particularly in HBV/HCV co-infection (26, 27).
However,
the study also highlights the limitations of AFP as a sole biomarker,
particularly in distinguishing between benign and malignant liver conditions in
co-infected individuals. The modest correlations between AFP and LFTs in the
HBV/HIV co-infected group suggest that AFP alone may not be sufficient to fully
capture the complexity of liver disease in these patients (28, 29). This
finding aligns with existing literature, which advocates for the use of a
combination of biomarkers and imaging techniques for a more comprehensive
assessment of liver health in co-infected individuals (30).
Our
study emphasizes the need for a nuanced understanding of AFP dynamics in HBV
co-infection contexts. The differential impact of HIV and HCV on AFP levels
reflects the underlying pathophysiological differences in how these viruses interact
with HBV and affect liver health. Future research should focus on elucidating
the specific mechanisms through which HIV and HCV modulate AFP production and
exploring the potential of AFP in combination with other biomarkers for
improved clinical management of co-infected patients (42-44).
Limitation
This
study has several limitations that should be considered. Firstly, the study
population was limited to patients from Warri, Delta State, Nigeria, which may
affect the generalizability of the findings to other regions with different
demographics or healthcare settings. Additionally, the study's reliance on
quarterly blood sample collections may have missed fluctuations in AFP levels
occurring between these intervals. The exclusion of individuals with
pre-existing liver disease or HCC may also limit the applicability of the
results to patients with more advanced liver conditions. Lastly, the study did
not account for potential variations in treatment regimens or adherence among
participants, which could influence AFP levels and liver function outcomes
Conclusion
The
study demonstrates that AFP levels are significantly higher in HBV patients
with HCV co-infection compared to those with HBV monoinfection
or HBV/HIV co-infection. This elevation in AFP suggests an increased risk of
liver disease progression and potential hepatocellular carcinoma (HCC) in the
HBV/HCV co-infection group. The observed positive correlations between AFP
levels and liver enzymes (ALT and AST) in the HBV/HCV group further indicate
ongoing liver damage and regeneration. These findings highlight the need for
vigilant monitoring and management of HBV patients with HCV co-infection to
address the heightened risk of liver complications.
Recommendations
1.
Enhanced Monitoring: Routine AFP and
liver function tests should be integrated into the care plans for HBV patients,
particularly those with HCV co-infection, to facilitate early detection of
liver disease progression and HCC.
2.
Differentiated Management
Strategies: Tailor treatment strategies based on co-infection status, with a
focus on more aggressive monitoring for HBV/HCV co-infected patients. For
HBV/HIV co-infected patients, emphasize maintaining immune function and monitoring
liver health through regular assessments.
3.
Public Health Initiatives:
Strengthen public health programs to enhance awareness about the risks of
co-infections and promote preventive measures, such as vaccination against HBV
and harm reduction strategies to prevent HCV transmission. Implementing comprehensive
screening programs can aid in early identification and intervention, improving
patient outcomes.
Further
Research: Future studies should explore the impact of various treatment
regimens and adherence on AFP levels and liver disease progression. Expanding
research to diverse populations and healthcare settings will help to validate
and generalize the findings.
Acknowledgments
We
extend our gratitude to the healthcare facilities and patients in Warri, Delta
State, for their participation and cooperation. Special thanks to the research
team for their dedication and hard work.
Author
contribution
MFO conceived the
study, and participated in manuscript review, and overall research supervision.
KFA participated in research design, data analysis, and manuscript
writing. ORO participated in research design, data collation and
manuscript writing. TBO participated in research design and sample
collection. TAM participated in sample collection, data collation and
manuscript writing. AWT participated in data analysis and manuscript
review. PNK participated in research design and data analysis. OJA
participated in research design and research supervision. OBO
participated in sample analysis, data analysis, data collation and manuscript
writing.
Conflict
of interest
The
authors declare that they have no competing interests.
Funding
There
is no funding agency involved in this research.
References
1. Hsu YC,
Huang DQ, Nguyen MH. Global burden of hepatitis B virus: current status, missed
opportunities and a call for action. Nat Rev Gastroenterol Hepatol.
2023;20(7):524–537.
2. Ajuwon BI, Yujuico I, Roper K, et
al. Hepatitis B virus infection in Nigeria: a systematic review and
meta-analysis of data published between 2010 and 2019. BMC Infect Dis. 2021;21:1120.
3. Hanif H,
Ali MJ, Susheela AT, et al. Update on the applications and limitations of
alpha-fetoprotein for hepatocellular carcinoma. World J Gastroenterol.
2022;28(2):216-229.
4. Adigun OO,
Yarrarapu SNS, Zubair M, et al. Alpha-fetoprotein
analysis. StatPearls (Internet). 2024 Jan- (Updated
2024 May 1).
5. Głowska-Ciemny J, Szymański M, Kuszerska
A, et al. The role of alpha-fetoprotein (AFP) in contemporary oncology: the
path from a diagnostic biomarker to an anticancer drug. Int J Mol Sci.
2023;24(3):2539.
6. Zhu AX,
Finn RS, Kang YK, et al. Serum alpha-fetoprotein and clinical outcomes in
patients with advanced hepatocellular carcinoma treated with ramucirumab. Br J
Cancer. 2021;124(8):1388–1397.
7. Tan A, Koh
S, Bertoletti A. Immune response in hepatitis B virus
infection. Cold Spring Harb Perspect Med. 2015;5(8):a021428.
8. Khanam A,
Chua JV, Kottilil S. Immunopathology of chronic
hepatitis B infection: role of innate and adaptive immune response in disease
progression. Int J Mol Sci. 2021;22(11):5497.
9. Zhao HJ,
Hu YF, Han QJ, Zhang J. Innate and adaptive immune escape mechanisms of
hepatitis B virus. World J Gastroenterol. 2022;28(9):881-896.
10. Maini MK,
Burton AR. Restoring, releasing or replacing adaptive immunity in chronic
hepatitis B. Nat Rev Gastroenterol Hepatol. 2019;16(10):662–675.
11. Revill PA,
Tu T, Netter HJ, et al. The evolution and clinical impact of hepatitis B virus
genome diversity. Nat Rev Gastroenterol Hepatol. 2020;17(11):618–634.
12. Cheng Z,
Lin P, Cheng N. HBV/HIV coinfection: impact on the development and clinical
treatment of liver diseases. Front Med (Lausanne). 2021;8:713981.
13. Ruta S,
Grecu L, Iacob D, Cernescu C, Sultana C. HIV-HBV
coinfection-current challenges for virologic monitoring. Biomedicines.
2023;11(5):1306.
14. Weldemhret L. Epidemiology and challenges of HBV/HIV
co-infection amongst HIV-infected patients in endemic areas: review. HIV AIDS (Auckl). 2021;13:485-490.
15. Hanif H,
Ali MJ, Susheela AT, et al. Update on the applications and limitations of
alpha-fetoprotein for hepatocellular carcinoma. World J Gastroenterol.
2022;28(2):216-229.
16. Zhu AX,
Finn RS, Kang YK, et al. Serum alpha-fetoprotein and clinical outcomes in
patients with advanced hepatocellular carcinoma treated with ramucirumab. Br J
Cancer. 2021;124(8):1388–1397.
17. Jasirwan COM, Fahira A, Siregar L, et al. The
alpha-fetoprotein serum is still reliable as a biomarker for the surveillance
of hepatocellular carcinoma in Indonesia. BMC Gastroenterol. 2020;20:215.
18. Cookey IT, Odenigbo KC, Okonkwo BJ, Okonko
IO. Prevalence of HBsAg among patients attending a tertiary hospital in Port
Harcourt, Nigeria. Int J Life Sci Res Arch. 2022;03(20):125-134.
19. Shea L,
Pesa J, Geonnotti G, et al. Improving diversity in
study participation: patient perspectives on barriers, racial differences and
the role of communities. Health Expect. 2022;25(4):1979-1987.
20. Akuta N,
Suzuki F, Kobayashi M, et al. Correlation between hepatitis B virus surface
antigen level and alpha-fetoprotein in patients free of hepatocellular
carcinoma or severe hepatitis. J Med Virol.
2014;86(1):131-8.
21. Hanif H,
Ali MJ, Susheela AT, et al. Update on the applications and limitations of
alpha-fetoprotein for hepatocellular carcinoma. World J Gastroenterol.
2022;28(2):216-229.
22. Bai DS,
Zhang C, Chen P, et al. The prognostic correlation of AFP level at diagnosis
with pathological grade, progression, and survival of patients with
hepatocellular carcinoma. Sci Rep. 2017;7:12870.
23. Lin K,
Huang Q, Zeng J, et al. Clinical significance of alpha-fetoprotein in
alpha-fetoprotein negative hepatocellular carcinoma underwent curative
resection. Dig Dis Sci. 2021;66(12):4545–4556.
24. Mavilia MG,
Wu GY. HBV-HCV coinfection: viral interactions, management, and viral
reactivation. J Clin Transl Hepatol.
2018;6(3):296-305.
25. Konstantinou
D, Deutsch M. The spectrum of HBV/HCV coinfection: epidemiology, clinical
characteristics, viral interactions and management. Ann Gastroenterol.
2015;28(2):221-228.
26. Desikan P,
Rangnekar A, Khan Z, et al. Sero-occurrence of HBV/HCV co-infection and levels
of liver enzymes among patients at a tertiary care hospital in central India: a
pilot study. Cent Asian J Glob Health. 2019;8(1):313.
27. Tseng CW,
Liu WC, Chen CY, et al. Impact of HCV viremia on HBV biomarkers in patients
coinfected with HBV and HCV. BMC Infect Dis. 2022;22:351.
28. Sherman KE,
Thomas DL. HIV and liver disease: a comprehensive update. Top Antivir Med. 2022;30(4):547-558.
29. Price JC,
Thio CL. Liver disease in the HIV-infected individual. *Clin Gastroenterol
Hepatol*. 2010;8(12):1002-12.
30. Xiao J,
Zhang Y, Wu J, et al. HIV/HBV coinfection: understanding the complex
interactions and their impact on spontaneous HBV clearance, chronic liver
damage, cirrhosis, and hepatocellular carcinoma. AIDS Rev. 2024;26(1):32-40.
31. Chamroonkul N, Bansal MB. HIV and the liver. Nat Rev
Gastroenterol Hepatol. 2019;16(1):1–2.
32. Gopalakrishna
H, Mironova M, Dahari H, et al. Advances and challenges in managing hepatitis D
virus: evolving strategies. Curr Hepatol Rep. 2024;23:32–44.
33. Klatte K,
Pauli-Magnus C, Love SB, et al. Monitoring strategies for clinical intervention
studies. Cochrane Database Syst Rev.
2021;12(12):MR000051.
34. Goorts K, Dizon J, Milanese S. The effectiveness of
implementation strategies for promoting evidence-informed interventions in
allied healthcare: a systematic review. BMC Health Serv
Res. 2021;21(1):241.
35. Carr BI.
Overview of clinical HCC and its management. Liver Cancer Middle East. 2021;7:123–134.
36. Fanning GC,
Zoulim F, Hou J, et al. Therapeutic strategies for
hepatitis B virus infection: towards a cure. Nat Rev Drug Discov.
2019;18(11):827–844.
37. Klaic M, Kapp S, Hudson P, et al. Implementability
of healthcare interventions: an overview of reviews and development of a
conceptual framework. Implement Sci. 2022;17(10).
38. Ricciardi
W, Cascini F. Guidelines and Safety Practices for
Improving Patient Safety. 2020 Dec 15. In: Donaldson L, Ricciardi W, Sheridan
S, et al., editors. Textbook of Patient Safety and Clinical Risk Management
(Internet). Cham (CH): Springer; 2021. Chapter 1.
39. Bolton RE, Bokhour BG, Hogan TP, Luger TM, Ruben M, Fix GM.
Integrating Personalized Care Planning into Primary Care: a
Multiple-Case Study of Early Adopting Patient-Centered
Medical Homes. J Gen Intern Med. 2020;35(2):428-436
40. Goetz LH,
Schork NJ. Personalized medicine: motivation, challenges, and progress. Fertil Steril. 2018 Jun;109(6):952-963.
41. Simmons LA,
Drake CD, Gaudet TW, Snyderman R. Personalized Health Planning in Primary Care
Settings. Fed Pract. 2016 Jan;33(1):27-34.
42. Shahzad,
M., Upshur, R., Donnelly, P. et al. A population-based approach to integrated
healthcare delivery: a scoping review of clinical care and public health
collaboration. BMC Public Health 19, 708 (2019).
43. Windle M,
Lee HD, Cherng ST, Lesko CR, Hanrahan C, Jackson JW, McAdams-DeMarco M,
Ehrhardt S, Baral SD, D'Souza G, Dowdy DW. From Epidemiologic Knowledge to
Improved Health: A Vision for Translational Epidemiology. Am J Epidemiol. 2019 Dec 31;188(12):2049-2060.
44. Trent RJ.
Public Health, Communicable Diseases and Global Health. Molecular Medicine.
2012:169–201.