The ketogenic diet as a promising
adjunctive therapy for glioma a comprehensive review
Arman Hamzei
1, Sara Hallaji 2, Seyed Farzin Hosseini 2, Arman
Keymoradzadeh 2 *
1 Neuroscience Research Center, School of Medicine, Guilan University
of Medical Science, Rasht, Iran
2 Department of Neurosurgery, Imam Hossein Hospital, Shahid Beheshti
University of Medical Science, Tehran, Iran
Corresponding
Authors: Arman Keymoradzadeh
* Email: keymoradzadeharman@gmail.com
Abstract
Gliomas are the most prevalent primary tumors of the brain and spinal
cord. Regrettably, the prognosis, especially for high-grade gliomas, remains
quite bleak. In recent decades, there's been a growing trend to replace or
combine radiotherapy with chemotherapy, targeted therapy, and personalized
treatment for different patients. For example, carboplatin and vincristine are
considered standard treatments for some patients with unresectable pediatric
low-grade gliomas. In recent years, ketogenic diet (KD) has emerged as a
promising investigational therapy for CNS tumors, with researchers exploring
its use in conjunction with existing treatment modalities. This review article
delves into the mechanisms underlying KD's potential therapeutic effects on
glioma and its efficacy, safety profile, and overall role in glioma treatment.
Keywords: Ketogenic Diet, Gliomas, Metabolism
Graphical abstract

Introduction
Brain
and other central nervous system (CNS) tumors, though uncommon, have a
significant impact on mortality and morbidity across all age groups. Despite
decades of research into the causes of brain and CNS tumors, no single risk
factor has been identified as a major contributor. These tumors are unique due
to their complex histological structure (1). Gliomas are
the most prevalent primary tumors of the brain and spinal cord. Regrettably,
the prognosis, especially for high-grade gliomas, remains quite bleak (2). Histologically, brain and CNS tumors share
characteristics of normal glial cells and are often named based on these
similarities. However, the exact origin of gliomas remains a topic of research,
with potential sources including normal glial cells, glial or neural
precursors, stem cells, or other cell types (3). Gliomas are
the most prevalent primary malignant brain tumors globally, often originating
from glial cells within the brain but also affecting other parts of the central
nervous system (CNS). The latest WHO classification categorizes diffuse gliomas
in adults into three main groups: astrocytoma IDH-mutant (grades 2, 3, or 4),
oligodendroglioma IDH-mutant and 1p/19q co-deleted (grades 2 or 3), and
glioblastoma (GBM) IDH-wildtype (grade 4). GBM, the most aggressive form,
carries a dismal prognosis with a median overall survival of less than two
years and a five-year survival rate of only 10% (4,5).
The
prognosis for WHO grade 1 and 2 gliomas is the most promising, with differences
based on molecular phenotype. IDH-mutant and 1p/19q co-deleted tumors
(oligodendroglioma) have the best prognosis, followed by IDH-mutant and 1p/19q
intact tumors, and then IDH-wildtype tumors. While a 'wait and see' approach
was previously considered safe for low-grade gliomas, recent trials suggest
that surgical resection should be performed as soon as possible to avoid tumor
progression and accurately identify molecular subtypes (6,7).
Patients
with GBM face a burdensome treatment regimen. Standard care involves surgical
resection of the tumor, followed by six to nine months of radiation therapy and
chemotherapy. Nevertheless, due to GBM's aggressive and highly vascular nature,
the disease frequently recurs within six months of treatment (8,9).
Repeated
cycles of standard therapy, including radiation therapy and temozolomide, are
often used to treat disease progression. However, these treatments can lead to
significant side effects, such as inflammation and edema in the brain (9–11). These
symptoms often precede seizures and other neurological complications, which can
adversely affect survival (10,12). Given the
limitations of current GBM therapies, researchers are seeking new and improved
treatments to prolong patient survival.
Scientists
have been exploring the link between cancer metabolism and treatment resistance
for nearly a century (13). In the 1950s,
Nobel Prize winner Otto Warburg made a significant contribution to the field of
cancer metabolism. Warburg discovered that cancer cells use a metabolic pathway
called "aerobic glycolysis" to generate energy from glucose, even in the
presence of oxygen (13,14). Warburg's
findings indicate that cancer cells employ metabolic pathways that prioritize
speed over efficiency, resulting from impaired mitochondrial respiration. This
implies that cancer cells may not have the capacity to metabolize ketones (12). Warburg's
findings are renowned and often cited in cancer research, and his conclusions
have been termed the "Warburg Effect." (12,14–18). This
discovery has been instrumental in the development of new cancer therapies,
including dietary interventions such as calorie restriction (CR) and the
ketogenic diet (KD) (15).
In
recent years, KD has emerged as a promising investigational therapy for CNS
tumors, with researchers exploring its use in conjunction with existing treatment
modalities.
(19). KD, or
'simulated fasting,' emerged around 1920 as a potential treatment for seizures.
Its origins can be traced back to ancient Greek physician Hippocrates, who used
fasting to manage seizure disorders. In 1911, French doctors Guelpa and Marie
formalized the use of fasting for epilepsy. By 1921, researchers suggested that
fasting and ketogenic diets could elevate ketone levels in healthy individuals,
leading to potential therapeutic benefits for children with epilepsy (20,21). A growing
body of research suggests that the ketogenic diet (KD) may be beneficial for
managing a wide range of health conditions, including neurological disorders
like epilepsy, migraine, Alzheimer's, motor neuron disease, autism, multiple
sclerosis, and Parkinson's, as well as non-neurological conditions such as
diabetes, obesity, cancer, acne, and polycystic ovary syndrome (22,23). Furthermore,
the ketogenic diet remains the primary treatment option for certain metabolic
disorders, including glucose transporter protein 1 (GLUT-1) deficiency
syndrome, complex 1 mitochondrial disorders (C1MDs), and pyruvate dehydrogenase
deficiency (24) (Figure 1).
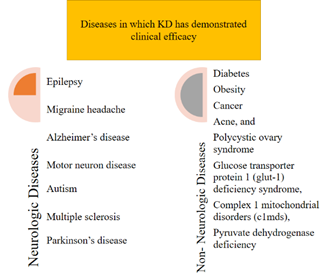
Figure
1.
Diseases in which KD has demonstrated clinical efficacy.
The
limited number of clinical trials investigating the ketogenic diet in GBM may
be due to concerns regarding the quality of life and well-being of terminally
ill patients, beyond diet tolerability and ketosis. Given the urgent need for
novel cancer therapies for GBM, this review aims to explore the feasibility and
efficacy of combining the ketogenic diet with standard GBM treatments. This
review delves into the mechanisms underlying the ketogenic diet's potential
therapeutic effects on glioma, as well as its efficacy, safety profile, and
overall role in glioma treatment.
Methods
Search
strategy
We
conducted a comprehensive search of the MEDLINE database using precise MeSH
terms such as “ketogenic diet” and
“glioblastoma,” “ketogenic diet” and “gliomas,” “calorie restriction” and
“glioblastoma,” “calorie restriction” and gliomas,” “diet intervention” and
“glioblastoma,” “diet intervention” and “gliomas,” and finally,
“low-carbohydrate diet” and “glioblastoma,” “low-carbohydrate diet” and
“gliomas”.
Study
selection and data extraction
We
saved and uploaded all initial studies identified through our search into
Mendeley software for title and abstract screening. Duplicate references were
removed during this process. All eligible studies were reviewed in full by the
author. We included only peer-reviewed, English-language articles published
between 2009 and 2019. Study designs included in vivo pre-clinical research,
patient case studies, randomized controlled trials, and retrospective studies
focusing on GBM treated with a KD. We excluded non-peer-reviewed articles
published before 2010. Our initial search yielded 126 results, which were
narrowed down to 75 eligible studies. To ensure the highest relevance, we
prioritized original research studies with a significant number of patients, providing
robust evidence for the KD's potential effectiveness in glioma treatment.
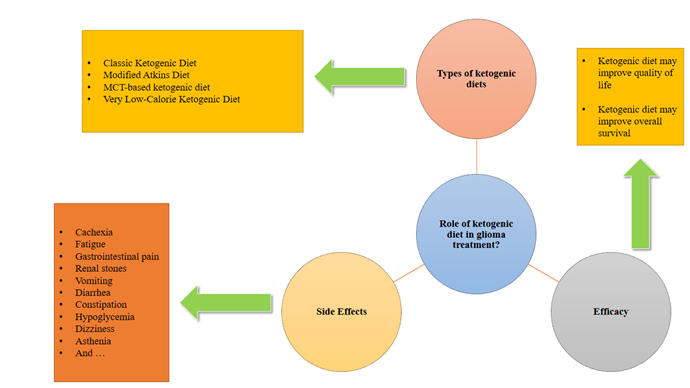
Variety
of Ketogenic Diets
There
are several types of KD used in clinical practice:
1.
The Classic
Ketogenic Diet (CKD)
The
Classic Ketogenic Diet (cKD) is a diet high in fat, very low in carbohydrates,
and moderate in protein. It has a high ketogenic ratio, meaning the ratio of
fats to carbohydrates and proteins is typically 3:1 or 4:1. This means you eat
significantly more fat than carbohydrates and proteins (22,25,26); This is an
isocaloric ketogenic diet (IKD) as defined by Trimboli et al. (26). Although the
Classic Ketogenic Diet (cKD) is effective, it can be difficult to follow for
both patients and their families. It requires strict adherence to a dietary
protocol that can be time-consuming to maintain. The cKD is especially
challenging for children, as they need to balance the ketogenic ratio with
their increasing energy and nutrient requirements. Food refusal and low
compliance can lead to inadequate nutrition and delayed growth in some children
(27,28).
2.
The Modified
Atkins Diet (MAD)
The
Modified Atkins Diet (MAD) is a variation of the original Atkins diet, created
in the 1970s to address the rising obesity rates. MAD allows for a higher
protein intake and doesn't require strict calorie counting. It also allows
people to start the diet without fasting (29). The MAD
offers increased flexibility and palatability by maintaining a 1:1 ratio of fat
to carbohydrates and protein. This means about 60% of calories come from fat,
30% from protein, and 10% from carbohydrates. MAD, along with the low glycemic
index diet (LGIT), are less restrictive alternatives to the KD because they
don't limit protein or calorie intake (22,25).
3.
Medium-Chain
Triglyceride (MCT)
Dr.
Peter Huttenlocher and his research team made a significant innovation to the
traditional KD by replacing some of the long-chain fats with medium-chain
triglycerides (MCTs). MCTs are absorbed more efficiently and transported
directly to the liver, leading to the production of more ketones per unit of
energy compared to long-chain fats. This allows for a reduction in the overall
fat content of the diet. While the traditional 4:1 ratio KD gets 90% of its
energy from fat, the MCT-based KD typically gets 70-75% of its energy from fat
(including MCTs and long-chain fats), allowing for more protein and
carbohydrates (22,23).
4.
The Very
Low-Calorie KD (VLCKD)
The
Very Low-Calorie KD (VLCKD) is a highly restrictive diet with a daily calorie
intake of 600 to 800 calories. This diet is followed for a short period of up
to 12 weeks and includes a minimum of 75 grams of protein per day. Carbohydrate
intake is very limited, 30 to 50 grams per day, while fat intake is fixed at 20
grams, primarily from olive oil and omega-3 sources. The diet is designed to
provide all necessary micronutrients according to European Food Safety
Authority (EFSA) guidelines (22,30).
Our
review indicates a dearth of comprehensive, standardized studies comparing the
effects of various ketogenic diets on glioma patients. This scarcity can be
attributed to the limited number of available randomized controlled trials on
this topic.
How
Ketogenic Diets Target Cancer: Mechanisms of Action
The
ketogenic diet exerts its therapeutic effects through several mechanisms:
cellular metabolic alterations, systemic and local inflammation reduction,
decreased reactive oxygen species (ROS), modulation of oncogenes and tumor
suppressors, and epigenetic modulation. These mechanisms will be discussed in
detail in the following sections.
Metabolic
Targets
Cancer
cells reprogram their metabolism to meet the heightened energy demands of rapid
growth and proliferation, a phenomenon known as the Warburg effect (13). Ketogenic
diets (KD) have demonstrated the potential to exert antitumor effects by targeting
both intracellular and extracellular metabolic pathways. KD induces a
decrease in blood glucose levels. Moreover, evidence suggests that it reduces
insulin and IGF-1 levels, consequently inhibiting the anabolic signaling of the
mTOR pathway (31,32). Intracellularly,
ketone bodies, particularly beta-hydroxybutyrate, have multifaceted effects.
Beta-hydroxybutyrate can be converted to acetyl-CoA, entering the Krebs cycle
and supporting energy production in healthy cells. However, in neoplastic
cells, impaired mitochondrial function prevents the efficient utilization of
acetyl-CoA for ATP generation. As a result, acetyl-CoA may be redirected
towards lipogenesis and cholesterol synthesis (33). Additionally,
ketone bodies can competitively inhibit monocarboxylate transporters, leading
to increased intracellular lactate levels and potentially affecting cancer cell
growth and survival (34). Pyruvate
kinase, a key glycolytic enzyme, is another intracellular target of KD in
tumors. The M2 isoform of pyruvate kinase is overexpressed in cancer cells and
contributes to their metabolic advantage (35,36). KD has been
shown to inhibit the expression of this isoform, reducing energy production and
promoting apoptosis in glioblastoma cells. Furthermore, KD downregulates other
key glycolytic enzymes, such as hexokinase, lactate dehydrogenase, and pyruvate
dehydrogenase, as well as the GLUT-1 transporter (37). In
glioblastoma mouse models, KD has been shown to reduce the expression of HIF-1α
and VEGF receptor 2, inhibiting angiogenesis and limiting tumor metabolic
changes (38). KD also
modifies the expression of AQP-4 and zonula occludens-1, reducing peritumoral
edema (38) (Figure 2).
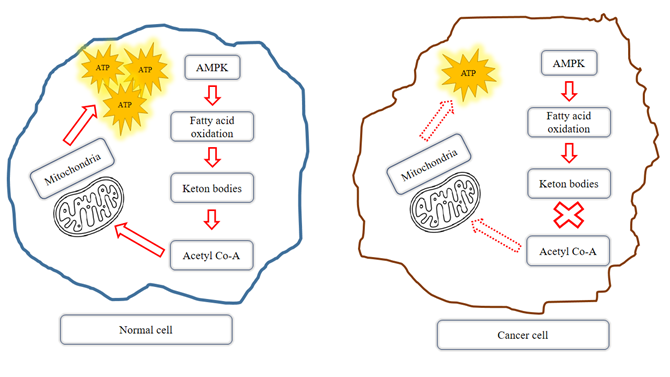
Figure
2. In
normal cells on a ketogenic diet (KD), lower glucose levels lead to an increase
in ketone bodies due to rising free fatty acids. This increases the level of
acetyl-CoA in mitochondria, which is used to produce energy (ATP). However, in
cancer cells on a KD, glycolysis is reduced, and mitochondria may be
dysfunctional, hindering their ability to produce ATP (90). AMPK; Adenosine
monophosphate-activated protein kinase.
Inflammation
Inflammation
has emerged as a hallmark of cancer, characterized by increased local and
systemic release of pro-inflammatory cytokines. This chronic inflammatory state
is often driven by the hyperactivation of NF-κB and other transcription
factors, promoting tumorigenesis and progression (39). Fatty acids,
by activating the PPAR-alpha receptor, can inhibit the NF-κB signaling pathway,
leading to downregulation of COX-2 and NOS, which are overexpressed in many
tumors (40). Furthermore,
ketogenic diets (KD), alone or in combination with the glutamine antagonist
6-diazo-5-oxo-1-norleucine (DON), have been shown to decrease TNF-α expression
in glioblastoma models, reducing tumor growth, inflammation, and prolonging
survival (41). The
inflammasome, a multiprotein complex, plays a pivotal role in initiating
inflammatory responses to pathogens or cellular damage, including cancer (42). Inhibition of
the inflammasome has been shown to reduce tumor growth and prolong survival in
glioma mouse models (43).
Beta-hydroxybutyrate, a ketone body produced during KD, can inhibit NLRP3
inflammasome assembly and subsequent cytokine production, reducing inflammatory
markers in central nervous system tumors (44). From an
immunotherapeutic perspective, KD has been shown to enhance anti-tumor immune
responses in glioblastoma mouse models. Specifically, KD can increase cytokine
production and CD8+ T cell-mediated cytolysis, promote CD4+ T cell infiltration
while maintaining normal levels of regulatory T cells, and decrease the
expression of co-inhibitory molecules CD86 and PD-L1, thereby reducing
tumor-mediated immunosuppression (45). Additionally,
KD has been shown to reduce peritumoral edema and steroid requirements (17,38,46).
Reactive
Oxygen Species (ROS)
To
support their growth, cancer cells undergo mitochondrial alterations that lead
to increased production of reactive oxygen species (ROS) (37). While this
ROS production and oxidative stress provide an evolutionary advantage for
cancer cells by increasing mutation rates and generating diverse clones, it
also poses a significant risk. If ROS levels exceed a critical threshold, the
resulting oxidative damage can overwhelm the cancer cell's repair mechanisms,
leading to cell death (47). This is the
underlying principle of conventional therapies like radiotherapy and
chemotherapy, which aim to induce irreparable damage in cancer cells (48). Ketogenic
diets, by limiting glucose-6-phosphate availability, disrupt both glycolysis
and the pentose phosphate pathway. The latter pathway is crucial for cancer
cells as it provides NADPH, a cofactor essential for maintaining reduced
glutathione levels and mitigating oxidative stress. However, this pathway also
supports the synthesis of nucleotides, thus limiting tumor growth and
proliferation (49). Interestingly,
while ketogenic diets increase oxidative stress in cancer cells, they
simultaneously promote an antioxidant response in healthy tissues.
Beta-hydroxybutyrate, a ketone body produced during ketosis, can activate
uncoupling protein 2 (UCP-2) in mitochondria, enhancing the cell's antioxidant
capacity (50). In
summary, ketogenic diets exhibit a dual effect: they synergize with
conventional therapies by increasing oxidative stress in cancer cells, while
simultaneously protecting healthy tissues through their antioxidant properties (51,52).
Epigenetic
Modulation
The
impact of ketogenic diets on the genome and gene expression is a relatively new
and understudied area. Ketogenic diets may modulate gene expression both
directly, by regulating DNA methylation (ketones increase adenosine levels,
inhibiting DNA methylation (53), and
indirectly, by altering histone modifications such as acetylation, methylation,
phosphorylation, ubiquitination, and lysine beta-hydroxybutyrylation; the
latter modification seems unique to ketone bodies. These epigenetic
modifications could explain the ketogenic diet's ability to positively
influence the expression of oncogenes and tumor suppressors (33,54,55). Another less
explored but scientifically intriguing topic is the role of microRNAs (miRNAs).
These small non-coding RNAs can regulate gene expression by binding to
complementary mRNA sequences, leading to their degradation and silencing.
MiRNAs are implicated in various pathological conditions, including cancer,
where altered miRNA expression often results in upregulation of oncogenes and
downregulation of tumor suppressors (50). In
glioblastoma, specific miRNAs have been found to be dysregulated. For instance,
studies have identified 256 significantly overexpressed miRNAs (primarily
miR-10b, miR-17-92 clusters, miR-21, and miR-93) and 95 significantly
under-expressed miRNAs (such as miR-7, miR-34a, miR-128, and miR-137) compared
to healthy brain tissue (56). In
glioblastoma mouse models, ketogenic diets have been shown to modulate the
expression of various miRNAs, reducing tumor progression and increasing
long-term survival (55,57,58).
Oncogenes
and Tumor Suppressors
Metalloproteinases,
a group of zinc-dependent endopeptidases, are responsible for breaking down the
extracellular matrix. Cancer cells often overexpress these enzymes to
facilitate local invasion and metastasis. Ketogenic diets have been shown to
reduce the expression of MMP-2 and MMP-9, as well as vimentin (38). P53, a
tumor suppressor gene, plays a crucial role in regulating cell proliferation,
apoptosis, and genomic stability. While p53 is typically expressed at low
levels and functions normally in healthy cells, it is often mutated and
overexpressed in cancer cells, contributing to therapeutic resistance.
Ketogenic diets can downregulate mutant p53 through deacetylation, inducing
apoptosis in neoplastic cells (55,59–61). AMP-activated
protein kinase (AMPK) is an enzyme that can activate tumor suppressors like
p53, inhibiting cell growth and cycle progression. Various compounds, including
metformin, curcumin, certain NSAIDs, and ketone bodies, have been shown to
activate AMPK (55,62). Comparisons
between ketogenic and standard diets in animal tumor models have revealed that
ketogenic diets can downregulate pathways mediated by IGF-1, PDGFR, and EGF,
which are frequently overexpressed in gliomas and activate Akt and mTOR. mTOR,
in turn, activates transcription factors such as HIF-1, upregulating oncogenes,
glucose transporters (GLUT), and glycolytic enzymes (51,63). Notably,
ketogenic diets enhance tumor response to PI3K inhibitors (64).
Effect
of KD on quality of life in patients with glioma tumors
Quality
of life (QOL) is a complex concept with various interpretations. It reflects an
individual's personal perspective on their life situation in relation to their
goals and expectations. QOL encompasses all aspects of life, including
psychological, social, and economic well-being, as well as relationships with
the environment. It's best understood as the difference between one's current
functional level and their ideal standard (65). A KD may
improve QOL by reducing chronic pain, inflammation, and enhancing metabolic
parameters (Table 1).
Table
1. Overview
of studies that have investigated effect of KD on quality of life in patients
with glioma tumors.
|
Reference |
Type of Glioma |
KD Duration |
Quality of Life |
|
(15) |
Astrocytoma
IDH-mutant grade III Astrocytoma
IDH-wild type grade IV |
14 months |
N/A |
|
(91) |
Astrocytoma IDH-mutant grade II-III Oligodendroglia IDH-mutant 1p/19q deleted grade II-III Astrocytoma IDH-wild type grade IV |
3 months |
Improved |
|
(86) |
Astrocytoma
IDH-mutant grade II-III Astrocytoma
IDH-wild type grade IV |
4 months |
Improved |
|
(88) |
Diffuse Midline glioma, high grade |
3 months |
Decreased |
|
(87) |
Astrocytoma
IDH-wildtype grade IV |
3.5 months |
Decreased |
|
(66) |
Diffuse Midline glioma, high grade |
6.5 months |
N/A |
|
(92) |
Astrocytoma
IDH-wild type grade IV |
6 to 26
months |
Improved |
|
(78) |
Astrocytoma IDH-wild type grade IV |
1 to 4 months |
Decreased |
|
(72) |
Astrocytoma
IDH-wild type grade IV |
1 to 12
months |
Improved |
The
Dark Side of KD: Potential Negative Effects and Risks
Adults
with malignant glioma who follow a KD may experience common side effects,
including gastrointestinal issues, weight loss, and a temporary rise in lipid
levels. Gastrointestinal symptoms like constipation, diarrhea, occasional
nausea, and vomiting are typically mild and often improve over time. These
effects can usually be managed through dietary adjustments, with guidance from
a dietitian or nutritionist. Medical intervention is rarely required (66,67). Consuming
smaller meals, increasing fiber intake, exercising, and drinking more fluids
can help prevent or alleviate these gastrointestinal issues. While weight loss
may be a desired outcome for overweight patients, those seeking to maintain or
gain weight should adjust their caloric intake accordingly (68). Weight loss,
particularly muscle mass loss (cachexia), is a significant concern in patients
with malignant glioma. Cachexia can reduce tolerance to cancer treatments,
impair lung function, and lead to lower survival rates. Research has shown that
very low-carbohydrate diets, which induce ketosis, can lower levels of serum
triglycerides, low-density lipoprotein, and total cholesterol while increasing
high-density lipoprotein cholesterol in adults (69,70). Restricting
carbohydrates and maintaining prolonged ketosis can lead to vitamin and mineral
deficiencies. Taking a daily multivitamin and mineral supplement can help
reduce the risk of these deficiencies (21,69,71). In one study,
hydro-electrolyte disorders were found (72) and in
another, a patient with an MTHFR mutation developed DVT (15). Studies
examining the use of KD in cancer patients have yielded varied results
regarding its impact on improving quality of life, cachexia, and fatigue (32,55).
Long-term
use of KD may lead to mild side effects, such as gastrointestinal discomfort or
kidney stones. These effects are often associated with medium-chain
triglyceride (MCT) oils and can be minimized by consuming the KD in limited
amounts and during specific timeframes, especially during radio-chemotherapy.
To ensure adherence to the KD, strong commitment and cooperation from both the
patient and their family are crucial for maintaining dietary-induced ketosis (63,73). Tracking and
maintaining adherence to a KD is essential for evaluating its effectiveness. In
adults, methods for measuring adherence beyond self-reporting include frequent
testing of serum β-hydroxybutyrate or urine acetoacetate levels during the
initial weeks on the diet, along with keeping detailed records of dietary
intake (71,74)
(Table 2) (Figure 3).
Table
2. Summary
of Side Effects of Ketogenic Diet.
|
Study |
Side Effects |
|
Rieger 2014 (78) |
Weight loss,
diarrhea, constipation, hunger |
|
Champ 2014 (15) |
Constipation, asthenia, weight
loss, nephrolithiasis, hypoglycemia |
|
Martin-McGill
2018 (91) |
Constipation |
|
Van der Louw
2019 (88) |
Hypoglycemia, hyperketosis,
vomiting, refusal to eat, asthenia, constipation |
|
Van der Louw
2019 (87)] |
Constipation,
nausea/vomiting, hypercholesterolemia, hypoglycemia, diarrhea, low
carnitine concentration |
|
Martin-McGill
2020 (72) |
Hypokalemia, hypocalcemia,
hypernatremia, hyperkalemia, constipation |
|
Panhans 2020 (86) |
Asthenia,
weight loss, nausea, vomiting, headache, decreased appetite |
|
Perez 2021 (66) |
Hypoglycemia, constipation,
hyperketosis, vomiting, asthenia, hyperuricemia |
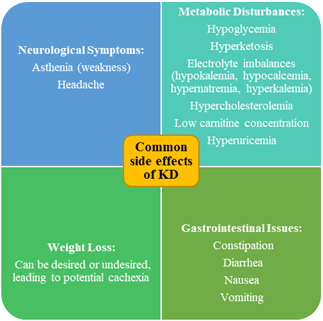
Figure 3. Summary of Side Effects of Ketogenic Diet.
Discrepancies
Between Preclinical and Clinical Findings
Pre-Clinical
Studies (Table
3)
Table
3. Summary
of some preclinical effects on glioblastoma and other brain tumors.
|
Study |
Summary of pre-clinical study Findings |
|
(75,76) |
Dietary
interventions like fasting and ketogenic diet can modulate metabolic pathways
and suppress tumor growth. |
|
(77,78) |
3:1 KD in glioblastoma models showed inconsistent results. |
|
(54,79) |
4:1 or 6:1 KD
improved survival in glioblastoma models, alone or with RT. |
|
(78) |
KD combined with bevacizumab showed synergistic effects in
glioblastoma models. |
|
(51) |
KD, alone or
with RT and temozolomide, prolonged survival and slowed tumor growth in
glioblastoma models. |
|
(80) |
Higher fat-to-carb ratio in KD did not benefit medulloblastoma
models. |
|
(41,81) |
KD and
caloric restriction reduced tumor growth and improved survival in
glioblastoma models. KD combined with DON had synergistic effects. |
|
(82) |
KD induced metabolic stress in tumor cells but spared healthy
tissue. |
|
(83) |
Gliomas can
adapt to ketogenic state, challenging the metabolic rigidity hypothesis. |
|
(76,84) |
Meta-analyses showed survival benefit of KD in various animal
tumor models. |
|
(85) |
Impact of IDH
mutations on KD response is unclear. Further research needed to identify
predictive genetic markers. |
Animal
models have demonstrated that dietary interventions, such as fasting and the
ketogenic diet, can modulate metabolic pathways and suppress tumor growth in
the brain (75,76).
While
some preclinical studies have explored the potential of a 3:1 KD in
glioblastoma mouse models, the results have been inconsistent, with some
studies failing to show significant therapeutic benefits (77,78). A higher
fat-to-carbohydrate ratio of 4:1 or 6:1 in the ketogenic diet (KD) has
demonstrated enhanced survival benefits in glioblastoma model (54,79) and in combination with RT (14). The combination
of the ketogenic diet (KD) and bevacizumab has shown a synergistic effect,
leading to increased survival, reduced tumor volume, and decreased ATP
concentration in glioblastoma models (78). Further
supporting the therapeutic potential of the ketogenic diet (KD), preclinical
studies have shown that KD can enhance survival and reduce tumor growth in
glioblastoma models, both as a standalone therapy and in combination with
conventional treatments like radiotherapy (RT) and temozolomide
(51).
While
a higher fat-to-carbohydrate ratio in the KD has shown promise in glioblastoma,
similar dietary modifications did not yield significant benefits in
medulloblastoma mouse models (80). Preclinical
studies have shown that the ketogenic diet (KD) and caloric restriction can
have significant anti-tumor effects in glioblastoma. Moreover, combining KD
with the chemotherapeutic agent DON can potentiate these effects, resulting in
improved survival, reduced tumor growth, and decreased inflammation and edema
(41,81).
A
recent preclinical study highlighted the metabolic advantages of the ketogenic
diet (KD) over a standard diet (SD) in high-grade glioma models. KD induced
metabolic stress in tumor cells by limiting the availability of essential amino
acids and impairing their ability to utilize ketone bodies for energy
production. In contrast, healthy brain tissue was able to adapt to the
ketogenic state and maintain normal energy metabolism (82).
Despite
promising preclinical results, the efficacy of the ketogenic diet (KD) in
treating brain tumors remains inconsistent. A 2016 study challenged the
hypothesis of metabolic rigidity in brain tumors, demonstrating that gliomas
can adapt to a ketogenic state by upregulating MCT1 and utilizing ketone
bodies. This finding highlights the complex interplay between tumor metabolism
and dietary interventions, underscoring the need for further research to
elucidate the underlying mechanisms and optimize KD therapy (83). Meta-analyses
of preclinical studies have shown a survival benefit associated with the
ketogenic diet (KD) in various animal tumor models, including brain tumors (76,84). Although the
impact of specific genetic mutations on the efficacy of the ketogenic diet (KD)
remains largely unexplored, studies on IDH mutations in gliomas have not
revealed significant differences in KD response. Further research is needed to
identify potential genetic markers that may predict patient response to KD
therapy (85).
Clinical
Studies (Table 4)
Table
4. Summary
of some clinical effects on glioblastoma and other brain tumors.
|
Study |
Patients |
Intervention |
Key Findings |
|
(78) |
20 with
recurrent glioblastoma |
KD + standard
therapy |
Longer PFS
with stable ketosis, improved PFS with KD+bevacizumab |
|
(15) |
53 with high-grade glioma |
KD + standard therapy |
Improved glucose control, potential for enhanced treatment
response |
|
(86) |
12 with
various glioma types |
3:1 KD +
standard therapy |
Improved
quality of life, reduced seizures, potential tumor response |
|
(63) |
Various grades of glioma |
MAD + standard therapy |
Improved seizure control, better quality of life |
|
(87) |
12 with
glioblastoma |
MAD or MCT
diet + standard therapy |
Positive
impact of MAD on glucose homeostasis |
|
(88) |
3 with DIPG |
KD + standard therapy |
Safe and well-tolerated, but limited sample size for survival
assessment |
|
Ongoing
Trials |
Various
glioma types |
KD + standard
therapy |
Currently
investigating the impact of KD on gliomas |
DIPG:
diffuse intrinsic pontine glioma.
Recent
clinical trials have explored the use of the ketogenic diet (KD) in treating
CNS tumors, particularly glioblastoma. The ERGO study, for instance, evaluated
20 patients with recurrent glioblastoma who received KD therapy. While all
patients experienced disease progression, those who maintained stable ketosis
demonstrated a longer progression-free survival (PFS) compared to those with
unstable ketosis. Moreover, combining KD with bevacizumab resulted in a longer
PFS compared to bevacizumab monotherapy (78).
A
retrospective study in 2014 explored the safety and tolerability of combining
the ketogenic diet (KD) with standard therapies in 53 patients with high-grade
gliomas. Six patients concurrently followed a KD, demonstrating its feasibility
and potential benefits. By reducing serum glucose levels, even in patients
receiving high-dose steroids, KD may enhance the efficacy of standard
treatments and improve patient outcomes (15).
A
2020 study demonstrated the positive impact of the ketogenic diet (KD) on the
quality of life of patients with various glioma types. Twelve patients treated
with standard therapy and a 3:1 KD reported improvements in energy levels,
mood, neurocognitive function, and overall well-being, along with reduced
seizure frequency. Furthermore, imaging studies suggested a potential tumor
response to the combined therapy of KD and standard treatment (86). A study
exploring the use of the Modified Atkins Diet (MAD) in combination with
standard therapy for various grades of glioma demonstrated its potential to
improve seizure control and patient quality of life (63).
The
2020 KEATING study and a 2019 study investigated the effects of different
dietary interventions on glioblastoma patients. While the KEATING study
demonstrated the positive impact of a Modified Atkins Diet (MAD) on glucose
homeostasis, the 2019 study, which used a 4:1 ketogenic diet (KD), failed to
show significant improvements in quality of life, neurological function, or
survival (87).
A
pilot study investigated the feasibility and safety of combining a ketogenic
diet with standard therapy in three children with diffuse intrinsic pontine
glioma (DIPG). Although the diet was well-tolerated, the small sample size
limited the ability to assess its impact on survival (88). These
findings were further corroborated by a more recent review of diffuse intrinsic
pontine glioma in children (66).
A
recent meta-analysis assessed the potential benefits of combining the ketogenic
diet (KD) with standard therapies for gliomas. While some studies suggested
improved overall survival, the small sample sizes, inclusion of various glioma
types, and absence of a control group limit the strength of these findings and
warrant further investigation (67). Ongoing
clinical trials are actively investigating the impact of KD on gliomas (89).
Conclusion
The
majority of preclinical and clinical data analyzed in this review suggest that
the KD
can
positively impact the treatment of central nervous system (CNS) tumors. By
targeting tumor metabolism, inflammation, gene expression, and the tumor
microenvironment, KD offers a promising adjuvant therapy. KD offers
several advantages, including low toxicity, affordability, and ease of
implementation. However, potential side effects and poor compliance can lead to
significant dropout rates. Additionally, the metabolic plasticity of cancer
cells, which allows them to adapt to different metabolic conditions, remains a
concern.
In
the near future, KD may be proposed as a combination therapy with conventional
chemotherapy (CT) and radiotherapy (RT). This combination therapy could offer
synergistic toxicity toward cancer cells and potential protection of healthy
cells from the toxic effects of standard therapies by increasing cellular
oxidative stress. To strengthen the evidence for KD's efficacy in treating CNS
tumors, larger-scale clinical trials are essential. Oncology departments should
collaborate with nutrition experts to integrate KD into conventional treatment
regimens. Additionally, both in vivo and in vitro studies are necessary to
definitively elucidate the cellular responses to the ketogenic environment and
uncover the underlying mechanisms of its therapeutic effects.
Acknowledgements
The
authors would like to thank the Professor Reza Shakib, immunologist of Guilan
university of medical science.
Author
contribution
AH: Search and
compilation of content, Writing the initial draft. SH: Scientific
editing of the neurology section of draft. SFH: Scientific editing of
the neurology section of draft. AK: Idea provider, Writing the
final draft, Image designer, General Scientific editing.
Conflict
of interest
There
is no Conflicts of interest/competing interests.
Funding
Self-funding.
References
1. Louis DN, Perry A, Reifenberger G, Von
Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health
Organization classification of tumors of the central nervous system: a summary.
Acta Neuropathol. 2016;131:803–20.
2. Ostrom QT, Francis SS, Barnholtz-Sloan JS.
Epidemiology of brain and other CNS tumors. Curr Neurol Neurosci Rep.
2021;21:1–12.
3. Chen R, Smith-Cohn M, Cohen AL, Colman H.
Glioma subclassifications and their clinical significance. Neurotherapeutics.
2017;14(2):284–97.
4. Ostrom QT, Patil N, Cioffi G, Waite K,
Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and
other central nervous system tumors diagnosed in the United States in
2013–2017. Neuro Oncol. 2020;22(Supplement_1):iv1–96.
5. Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of
the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–51.
6. Duffau H, Taillandier L. New concepts in the
management of diffuse low-grade glioma: proposal of a multistage and
individualized therapeutic approach. Neuro Oncol. 2015;17(3):332–42.
7. Weller M, Le Rhun E. How did lomustine
become standard of care in recurrent glioblastoma? Cancer Treat Rev.
2020;87:102029.
8. Zuccoli G, Marcello N, Pisanello A, Servadei
F, Vaccaro S, Mukherjee P, et al. Metabolic management of glioblastoma
multiforme using standard therapy together with a restricted ketogenic diet:
Case Report. Nutr Metab (Lond). 2010;7:1–7.
9. Chinot OL, Wick W, Mason W, Henriksson R,
Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy–temozolomide for
newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–22.
10. Duan C, Yang R, Yuan L, Engelbach JA, Tsien
CI, Rich KM, et al. Late effects of radiation prime the brain microenvironment
for accelerated tumor growth. Int J Radiat Oncol Biol Phys. 2019;103(1):190–4.
11. Taal W, Brandsma D, de Bruin HG, Bromberg JE,
Swaak‐Kragten AT, Sillevis Smitt PAE, et al. Incidence of early
pseudo‐progression in a cohort of malignant glioma patients treated with
chemoirradiation with temozolomide. Cancer. 2008;113(2):405–10.
12. Woodhouse C, Ward T, Gaskill-Shipley M,
Chaudhary R. Feasibility of a modified Atkins diet in glioma patients during
radiation and its effect on radiation sensitization. Curr Oncol.
2019;26(4):e433.
13. Warburg O, Wind F, Negelein E. The metabolism
of tumors in the body. J Gen Physiol. 1927;8(6):519.
14. Abdelwahab MG, Fenton KE, Preul MC, Rho JM,
Lynch A, Stafford P, et al. The ketogenic diet is an effective adjuvant to
radiation therapy for the treatment of malignant glioma. PLoS One.
2012;7(5):e36197.
15. Champ CE, Palmer JD, Volek JS, Werner-Wasik M,
Andrews DW, Evans JJ, et al. Targeting metabolism with a ketogenic diet during
the treatment of glioblastoma multiforme. J Neurooncol. 2014;117:125–31.
16. Zhang C, Liu J, Liang Y, Wu R, Zhao Y, Hong X,
et al. Tumour-associated mutant p53 drives the Warburg effect. Nat Commun.
2013;4(1):2935.
17. Elsakka AMA, Bary MA, Abdelzaher E, Elnaggar
M, Kalamian M, Mukherjee P, et al. Management of glioblastoma multiforme in a
patient treated with ketogenic metabolic therapy and modified standard of care:
a 24-month follow-up. Front Nutr. 2018;5:20.
18. Artzi M, Liberman G, Vaisman N, Bokstein F,
Vitinshtein F, Aizenstein O, et al. Changes in cerebral metabolism during
ketogenic diet in patients with primary brain tumors: 1 H-MRS study. J
Neurooncol. 2017;132:267–75.
19. Poff A, Koutnik AP, Egan KM, Sahebjam S,
D’Agostino D, Kumar NB. Targeting the Warburg effect for cancer treatment:
Ketogenic diets for management of glioma. In: Seminars in Cancer Biology.
Elsevier; 2019. p. 135–48.
20. Cervenka MC, Wood S, Bagary M, Balabanov A,
Bercovici E, Brown M-G, et al. International recommendations for the management
of adults treated with ketogenic diet therapies. Neurol Clin Pract.
2021;11(5):385–97.
21. Paoli A, Rubini A, Volek JS, Grimaldi KA.
Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate
(ketogenic) diets. Eur J Clin Nutr. 2013;67(8):789–96.
22. Dal Bello S, Valdemarin F, Martinuzzi D,
Filippi F, Gigli GL, Valente M. Ketogenic diet in the treatment of gliomas and
glioblastomas. Nutrients. 2022;14(18):3851.
23. McDonald TJW, Cervenka MC. Ketogenic diets for
adult neurological disorders. Neurotherapeutics. 2018;15(4):1018–31.
24. Kossoff EH, Zupec‐Kania BA, Auvin S,
Ballaban‐Gil KR, Christina Bergqvist AG, Blackford R, et al. Optimal clinical
management of children receiving dietary therapies for epilepsy: Updated
recommendations of the International Ketogenic Diet Study Group. Epilepsia
open. 2018;3(2):175–92.
25. Freeman JM, Kossoff EH, Rubenstein JE, Zahava
Turner RD. Ketogenic diets: treatments for epilepsy and other disorders. Demos
Medical Publishing; 2011.
26. Trimboli P, Castellana M, Bellido D, Casanueva
FF. Confusion in the nomenclature of ketogenic diets blurs evidence. Rev Endocr
Metab Disord. 2020;21(1):1–3.
27. Ferraris C, Guglielmetti M, Pasca L, De
Giorgis V, Ferraro OE, Brambilla I, et al. Impact of the ketogenic diet on
linear growth in children: a single-center retrospective analysis of 34 cases.
Nutrients. 2019;11(7):1442.
28. Perna S, Ferraris C, Guglielmetti M, Alalwan
TA, Mahdi AM, Guido D, et al. Effects of classic ketogenic diet in children
with refractory epilepsy: a retrospective cohort study in Kingdom of Bahrain.
Nutrients. 2022;14(9):1744.
29. Kossoff EH. The modified atkins diet for
epilepsy: two decades of an “Alternative” ketogenic diet therapy. Pediatr
Neurol. 2023;
30. Caprio M, Infante M, Moriconi E, Armani A,
Fabbri A, Mantovani G, et al. Very-low-calorie ketogenic diet (VLCKD) in the
management of metabolic diseases: systematic review and consensus statement
from the Italian Society of Endocrinology (SIE). J Endocrinol Invest.
2019;42:1365–86.
31. McDonald TJW, Cervenka MC. The expanding role
of ketogenic diets in adult neurological disorders. Brain Sci. 2018;8(8):148.
32. Barrea L, Caprio M, Tuccinardi D, Moriconi E,
Di Renzo L, Muscogiuri G, et al. Could ketogenic diet “starve” cancer? Emerging
evidence. Crit Rev Food Sci Nutr. 2022;62(7):1800–21.
33. Dąbek A, Wojtala M, Pirola L, Balcerczyk A.
Modulation of cellular biochemistry, epigenetics and metabolomics by ketone
bodies. Implications of the ketogenic diet in the physiology of the organism
and pathological states. Nutrients. 2020;12(3):788.
34. Poff AM, Ari C, Arnold P, Seyfried TN,
D’agostino DP. Ketone supplementation decreases tumor cell viability and
prolongs survival of mice with metastatic cancer. Int J cancer.
2014;135(7):1711–20.
35. Wong N, Ojo D, Yan J, Tang D. PKM2 contributes
to cancer metabolism. Cancer Lett. 2015;356(2):184–91.
36. Luo W, Hu H, Chang R, Zhong J, Knabel M,
O’Meally R, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for
hypoxia-inducible factor 1. Cell. 2011;145(5):732–44.
37. Ji C-C, Hu Y-Y, Cheng G, Liang L, Gao B, Ren
Y-P, et al. A ketogenic diet attenuates proliferation and stemness of glioma
stem-like cells by altering metabolism resulting in increased ROS production.
Int J Oncol. 2019;56(2):606–17.
38. Woolf EC, Curley KL, Liu Q, Turner GH,
Charlton JA, Preul MC, et al. The ketogenic diet alters the hypoxic response
and affects expression of proteins associated with angiogenesis, invasive
potential and vascular permeability in a mouse glioma model. PLoS One.
2015;10(6):e0130357.
39. Aggarwal BB, Shishodia S, Sandur SK, Pandey
MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol.
2006;72(11):1605–21.
40. Cullingford TE. The ketogenic diet; fatty
acids, fatty acid-activated receptors and neurological disorders.
Prostaglandins, Leukot Essent Fat Acids. 2004;70(3):253–64.
41. Mukherjee P, Augur ZM, Li M, Hill C, Greenwood
B, Domin MA, et al. Therapeutic benefit of combining calorie-restricted
ketogenic diet and glutamine targeting in late-stage experimental glioblastoma.
Commun Biol. 2019;2(1):200.
42. Sharma BR, Kanneganti T-D. NLRP3 inflammasome
in cancer and metabolic diseases. Nat Immunol. 2021;22(5):550–9.
43. Li L, Liu Y. Aging-related gene signature
regulated by Nlrp3 predicts glioma progression. Am J Cancer Res. 2014;5(1):442.
44. Youm Y-H, Nguyen KY, Grant RW, Goldberg EL,
Bodogai M, Kim D, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3
inflammasome–mediated inflammatory disease. Nat Med. 2015;21(3):263–9.
45. Lussier DM, Woolf EC, Johnson JL, Brooks KS,
Blattman JN, Scheck AC. Enhanced immunity in a mouse model of malignant glioma
is mediated by a therapeutic ketogenic diet. BMC Cancer. 2016;16:1–10.
46. Seyfried TN, Shelton L, Arismendi-Morillo G,
Kalamian M, Elsakka A, Maroon J, et al. Provocative question: should ketogenic
metabolic therapy become the standard of care for glioblastoma? Neurochem Res.
2019;44:2392–404.
47. Vendramin R, Litchfield K, Swanton C. Cancer
evolution: Darwin and beyond. EMBO J. 2021;40(18):e108389.
48. Liu Y, Zheng C, Huang Y, He M, Xu WW, Li B.
Molecular mechanisms of chemo‐and radiotherapy resistance and the potential
implications for cancer treatment. MedComm. 2021;2(3):315–40.
49. Alfarouk KO, Ahmed SBM, Elliott RL, Benoit A,
Alqahtani SS, Ibrahim ME, et al. The pentose phosphate pathway dynamics in
cancer and its dependency on intracellular pH. Metabolites. 2020;10(7):285.
50. Fine EJ, Miller A, Quadros E V, Sequeira JM,
Feinman RD. Acetoacetate reduces growth and ATP concentration in cancer cell
lines which over-express uncoupling protein 2. Cancer Cell Int. 2009;9:1–11.
51. Scheck AC, Abdelwahab M, Stafford P, Kim D-Y,
Iwai S, Preul MC, et al. Mechanistic studies of the ketogenic diet as an
adjuvant therapy for malignant gliomas. Cancer Res. 2010;70(8_Supplement):638.
52. Klement RJ, Champ CE. Calories, carbohydrates,
and cancer therapy with radiation: exploiting the five R’s through dietary
manipulation. Cancer Metastasis Rev. 2014;33(1):217–29.
53. Boison D. New insights into the mechanisms of
the ketogenic diet. Curr Opin Neurol. 2017;30(2):187–92.
54. Stafford P, Abdelwahab MG, Kim DY, Preul MC,
Rho JM, Scheck AC. The ketogenic diet reverses gene expression patterns and
reduces reactive oxygen species levels when used as an adjuvant therapy for
glioma. Nutr Metab (Lond). 2010;7:1–11.
55. Talib WH, Mahmod AI, Kamal A, Rashid HM,
Alashqar AMD, Khater S, et al. Ketogenic diet in cancer prevention and therapy:
molecular targets and therapeutic opportunities. Curr Issues Mol Biol.
2021;43(2):558–89.
56. Møller HG, Rasmussen AP, Andersen HH, Johnsen
KB, Henriksen M, Duroux M. A systematic review of microRNA in glioblastoma
multiforme: micro-modulators in the mesenchymal mode of migration and invasion.
Mol Neurobiol. 2013;47:131–44.
57. Preston MJ, Stylianou J, Zeng MQ, Glover MS,
Scheck AC, Woolf MEC, et al. OP16. The ketogenic diet induces epigenetic
changes that play key roles in tumour development. Neuro Oncol. 2017;19(Suppl
1):i28.
58. Zeng Q, Stylianou T, Preston J, Glover S,
O’Neill K, Woolf EC, et al. The ketogenic diet alters the epigenetic landscape
of gbm to potentiate the effects of chemotherapy and radiotherapy. Neuro Oncol.
2019;21(Suppl 4):iv8.
59. Ozaki T, Nakagawara A. Role of p53 in cell
death and human cancers. Cancers (Basel). 2011;3(1):994–1013.
60. Yue X, Zhao Y, Xu Y, Zheng M, Feng Z, Hu W.
Mutant p53 in cancer: accumulation, gain-of-function, and therapy. J Mol Biol.
2017;429(11):1595–606.
61. Liu K, Li F, Sun Q, Lin N, Han H, You K, et
al. p53 β-hydroxybutyrylation attenuates p53 activity. Cell Death Dis.
2019;10(3):243.
62. Li W, Saud SM, Young MR, Chen G, Hua B.
Targeting AMPK for cancer prevention and treatment. Oncotarget.
2015;6(10):7365.
63. Strowd RE, Cervenka MC, Henry BJ, Kossoff EH,
Hartman AL, Blakeley JO. Glycemic modulation in neuro-oncology: experience and
future directions using a modified Atkins diet for high-grade brain tumors.
Neuro-oncology Pract. 2015;2(3):127–36.
64. Hopkins BD, Pauli C, Du X, Wang DG, Li X, Wu
D, et al. Suppression of insulin feedback enhances the efficacy of PI3K
inhibitors. Nature. 2018;560(7719):499–503.
65. Abboud M, AlAnouti F, Georgaki E, Papandreou
D. Effect of ketogenic diet on quality of life in adults with chronic disease:
A systematic review of randomized controlled trials. Nutrients.
2021;13(12):4463.
66. Perez A, van der Louw E, Nathan J, El‐Ayadi M,
Golay H, Korff C, et al. Ketogenic diet treatment in diffuse intrinsic pontine
glioma in children: Retrospective analysis of feasibility, safety, and survival
data. Cancer Rep. 2021;4(5):e1383.
67. Sargaço B, Oliveira PA, Antunes ML, Moreira
AC. Effects of the ketogenic diet in the treatment of gliomas: a systematic
review. Nutrients. 2022;14(5):1007.
68. Winter SF, Loebel F, Dietrich J. Role of
ketogenic metabolic therapy in malignant glioma: a systematic review. Crit Rev
Oncol Hematol. 2017;112:41–58.
69. Cervenka MC, Patton K, Eloyan A, Henry B,
Kossoff EH. The impact of the modified Atkins diet on lipid profiles in adults
with epilepsy. Nutr Neurosci. 2016;19(3):131–7.
70. Pinto A, Bonucci A, Maggi E, Corsi M, Businaro
R. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new
perspectives for neuroprotection in Alzheimer’s disease. Antioxidants.
2018;7(5):63.
71. Schoeler NE, Cross JH. Ketogenic dietary
therapies in adults with epilepsy: a practical guide. Pract Neurol.
2016;16(3):208–14.
72. Martin-McGill KJ, Marson AG, Tudur Smith C,
Young B, Mills SJ, Cherry MG, et al. Ketogenic diets as an adjuvant therapy for
glioblastoma (KEATING): a randomized, mixed methods, feasibility study. J
Neurooncol. 2020;147:213–27.
73. Klement RJ, Sweeney RA. Impact of a ketogenic
diet intervention during radiotherapy on body composition: I. Initial clinical
experience with six prospectively studied patients. BMC Res Notes. 2016;9:1–13.
74. Schwartz KA, Noel M, Nikolai M, Chang HT.
Investigating the ketogenic diet as treatment for primary aggressive brain
cancer: challenges and lessons learned. Front Nutr. 2018;5:11.
75. Seyfried TN, Sanderson TM, El-Abbadi MM,
McGowan R, Mukherjee P. Role of glucose and ketone bodies in the metabolic
control of experimental brain cancer. Br J Cancer. 2003;89(7):1375–82.
76. Lv M, Zhu X, Wang H, Wang F, Guan W. Roles of
caloric restriction, ketogenic diet and intermittent fasting during initiation,
progression and metastasis of cancer in animal models: a systematic review and
meta-analysis. PLoS One. 2014;9(12):e115147.
77. Maurer GD, Brucker DP, Bähr O, Harter PN,
Hattingen E, Walenta S, et al. Differential utilization of ketone bodies by
neurons and glioma cell lines: a rationale for ketogenic diet as experimental
glioma therapy. BMC Cancer. 2011;11:1–17.
78. Rieger J, Bähr O, Maurer GD, Hattingen E,
Franz K, Brucker D, et al. ERGO: A pilot study of ketogenic diet in recurrent
glioblastoma Erratum in/ijo/45/6/2605. Int J Oncol. 2014;44(6):1843–52.
79. Zhou W, Mukherjee P, Kiebish MA, Markis WT,
Mantis JG, Seyfried TN. The calorically restricted ketogenic diet, an effective
alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:1–15.
80. Dang MT, Wehrli S, Dang C V, Curran T. The
ketogenic diet does not affect growth of hedgehog pathway medulloblastoma in
mice. PLoS One. 2015;10(7):e0133633.
81. Morscher RJ, Aminzadeh-Gohari S, Feichtinger
RG, Mayr JA, Lang R, Neureiter D, et al. Inhibition of neuroblastoma tumor
growth by ketogenic diet and/or calorie restriction in a CD1-Nu mouse model.
PLoS One. 2015;10(6):e0129802.
82. Ciusani E, Vasco C, Rizzo A, Girgenti V,
Padelli F, Pellegatta S, et al. MR-spectroscopy and survival in mice with high
grade glioma undergoing unrestricted ketogenic diet. Nutr Cancer.
2021;73(11–12):2315–22.
83. De Feyter HM, Behar KL, Rao JU,
Madden-Hennessey K, Ip KL, Hyder F, et al. A ketogenic diet increases transport
and oxidation of ketone bodies in RG2 and 9L gliomas without affecting tumor
growth. Neuro Oncol. 2016;18(8):1079–87.
84. Klement RJ, Champ CE, Otto C, Kämmerer U.
Anti-tumor effects of ketogenic diets in mice: a meta-analysis. PLoS One.
2016;11(5):e0155050.
85. Javier R, Wang W, Drumm M, McCortney K,
Sarkaria JN, Horbinski C. The efficacy of an unrestricted cycling ketogenic
diet in preclinical models of IDH wild-type and IDH mutant glioma. PLoS One.
2022;17(2):e0257725.
86. Panhans CM, Gresham G, Amaral LJ, Hu J.
Exploring the feasibility and effects of a ketogenic diet in patients with CNS
malignancies: a retrospective case series. Front Neurosci. 2020;14:390.
87. van der Louw EJTM, Olieman JF, van den Bemt
PMLA, Bromberg JEC, Oomen-de Hoop E, Neuteboom RF, et al. Ketogenic diet
treatment as adjuvant to standard treatment of glioblastoma multiforme: a
feasibility and safety study. Ther Adv Med Oncol. 2019;11:1758835919853958.
88. van der Louw EJTM, Reddingius RE, Olieman JF,
Neuteboom RF, Catsman‐Berrevoets CE. Ketogenic diet treatment in recurrent
diffuse intrinsic pontine glioma in children: A safety and feasibility study.
Pediatr Blood Cancer. 2019;66(3):e27561.
89. Woolf EC, Scheck AC. The ketogenic diet for
the treatment of malignant glioma. J Lipid Res. 2015;56(1):5–10.
90. Cecchi N, Romanelli R, Ricevuti F, Amitrano M,
Carbone MG, Dinardo M, et al. Current knowledges in
pharmaconutrition:“Ketogenics” in pediatric gliomas. Front Nutr.
2023;10:1222908.
91. Martin-McGill KJ, Marson AG, Tudur Smith C,
Jenkinson MD. The modified ketogenic diet in adults with glioblastoma: an
evaluation of feasibility and deliverability within the National Health
Service. Nutr Cancer. 2018;70(4):643–9.
92. Klein P, Tyrlikova I, Zuccoli G, Tyrlik A,
Maroon JC. Treatment of glioblastoma multiforme with “classic” 4: 1 ketogenic
diet total meal replacement. Cancer Metab. 2020;8:1–11.