Predictive value
of hematologic indices in COVID-19 disease outcomes
Fatemeh Nejatifar 1, Ali Alavi Foumani 2,
Saman Maroufizadeh 3, Bardia Afsharian 4, Zahra Chegini 4, Amir Mohammad Ghanbari 4*
1 Associate professor, Hematology and Medical Oncology Department,
Guilan University of Medical Sciences, Rasht, Iran
2 Inflammatory Lung Diseases Research Center, Department of
Pulmonology, Guilan University of Medical Sciences, Rasht, Iran
3 Department of Biostatistics and Epidemiology, School of Health,
Guilan University of Medical Sciences, Rasht, Iran
4 Student Research Committee, Faculty of Medicine, Guilan University
of Medical Sciences, Rasht, Iran
Corresponding
Authors: Amir Mohammad Ghanbari
*
Email: amir.damash@yahoo.com
Abstract
Introduction: COVID-19 was
declared a worldwide concern for public health in January 2020 by the World
Health Organization. Most patients manifest mild symptoms. In more severe cases
it can lead to sepsis, acute respiratory distress syndrome and other organ
dysfunction. Lymphopenia, increased inflammatory markers and dysregulated liver
enzymes are observed in many patients and is related to higher mortality rates.
Materials and Methods: We evaluated two hundred and sixty-eight
patients in this study. All patients had dyspnea, and O2 saturation below 93%
and were tested positive for COVID-19 through RT-PCR. Patients’ demographic,
clinical and paraclinical information were obtained on admission and disease
outcomes were assessed based on these data. The evaluated indices were
previously shown to be altered in patients with different disease outcomes.
Results: From a total of 268 included patients, 40% had severe
disease, 29% were admitted to ICU, 22% required mechanical ventilation and 24%
died during hospitalization. WBC counts, neutrophil counts, NLR, serum LDH
activity and serum albumin levels were the most powerful factors in predicting
disease outcomes.
Conclusion: COVID-19 disease severity and outcomes were affected by
hematologic indices and laboratory results.
Keywords: COVID-19, Neutrophil, White blood cell, Neutrophil lymphocyte ratio
Introduction
COVID-19
was first seen in December 2019 in Wuhan, China and was declared a worldwide
concern for public health in January 2020 by World Health Organization (1). The disease was named “COVID-19”
in February 2020 and the virus was named as “SARS-CoV-2” (2). SARS-CoV-2 was the third
coronavirus in the past 20 years that can infect human species (3). About 81% of the patients manifest
mild symptoms, the symptoms can be severe in 14% of the patients and it can
lead to sepsis, acute respiratory distress syndrome and other organs’ failure
in 5% of the patients (4).
Previous
studies have shown lung involvement in CT-scan in most patients. Lymphopenia,
increased inflammatory markers (like ferritin and C-reactive protein) and
elevated AST and ALT levels are also observed in many patients (5, 6). It is shown that lymphopenia
presents despite normal white blood cell count and lymphocyte count is related
with disease severity and prognosis (7). Higher mortality rates are
observed among patients with lymphopenia, thrombocytopenia, elevated
inflammatory markers (like CRP, LDH and ferritin) and coagulopathies (8, 9).
As
mentioned, these hematologic indices and inflammatory markers are shown to have
a predictive role in determining the disease outcome. In this study we
evaluated this predictive role in COVID-19 patients.
Materials and methods
Two
hundred and sixty-eight patients were enrolled in this study. All patients were
admitted to Razi Hospital, Rasht from March 2021 until March 2022. All patients
had dyspnea, O2 saturation below 93% and were tested positive for
COVID-19 through RT-PCR. Patients with underlying medical condition (which is
known to affect blood cell counts or other evaluated lab data e.g. hematologic
malignancies) were excluded from this study. Demographic and clinical
information were gathered from patients’ admission records. A blood test was
administered in admission to evaluate hematologic and inflammatory indices.
Disease
severity was classified as moderate (90 < SPO2 < 94 or less
than 50% lung involvement in CT-Scan) and severe (SPO2 < 90 or
respiratory rate over 30 or PCO2/FIO2 < 300). The
patients were also classified by admission to intensive care unit, death within
hospital admission and requiring ventilation. Patients’ demographic data, past
medical records, inflammatory and hematologic indices in admission and clinical
presentation were assessed based on the mentioned categories.
In
this survey, quantitative data are shown as “mean (standard deviation)” and
qualitative data are shown as “frequency (percentile)”. Man-Whitney test was
done to compare the hematologic indices based on disease severity (moderate or
severe), ICU admission (yes or no), death within hospital admission (yes or no)
and requiring mechanical ventilation (yes or no). Area under the receiver
operating characteristics curve (AUC for ROC curve) was shown to evaluate the
potential of hematologic indices to predict disease severity, ICU admission,
death within hospital admission and requirement of mechanical ventilation. All
results were analyzed with a 95% confidence interval.
Results
Two
hundred and sixty-eight patients, who were admitted to Razi hospital with a
definite diagnosis of COVID-19, were enrolled in this study. 109 patients (41%)
were male and the mean age was 56 ± 16.6. 105 patients (39%) had hypertension,
75 patients (28%) had diabetes mellitus,30 patients (11%) and ischemic heart
disease, eleven patients (4%) had an underlying pulmonary disease and ten
patients (4%) had chronic kidney disease. The mean systolic blood pressure in
admission was 123.2 ± 19.4, the mean pulse rate and respiratory rate were 90.1
±12.8 and 23.2 ±4.4 and the mean O2 saturation was 89.9 ± 8.3. All clinical and
demographic data are shown in table 1.
Lab
test results in admission are also shown in table 2.
As
shown in table 3, 108 patients (40%) had severe disease, 78 patients (29%) were
admitted in ICU, 58 patients (22%) required mechanical ventilation and 63
patients (24%) died during hospitalization.
Table
1.
Patients' clinical and demographic data.
|
|
Frequency (percentage) or
mean ± standard deviation
|
|
Age (years)
|
56 ± 16.6
|
|
Gender
|
Male
|
109 (41%)
|
|
Female
|
159 (59%)
|
|
HTN
|
105 (39%)
|
|
DM
|
75 (28%)
|
|
CKD
|
10 (4%)
|
|
Pulmonary disease
|
11 (4%)
|
|
IHD
|
30 (11%)
|
|
Temperature
|
37.1 ± 0.5
|
|
Systolic blood pressure
|
123.2 ± 19.4
|
|
Diastolic blood pressure
|
76.1 ± 12.7
|
|
Pulse rate
|
90.1 ± 12.8
|
|
Respiratory rate
|
23.2 ± 4.4
|
|
O2 saturation
|
89.9 ± 8.3
|
Table
2.
Patients' lab test results in admission.
|
|
Mean ± SD
|
Median (IQR)
|
|
WBC (× 106/mL)
|
8.1 ± 4.3
|
7.1 (5.1 –
10)
|
|
Hb (g/dL)
|
12 ± 1.9
|
12.2 (10.8 – 13.2)
|
|
RDW (%)
|
14 ± 2
|
13.5 (12.7 – 14.8)
|
|
MCV (fL)
|
84 ± 7.9
|
85 (80.5 – 88.4)
|
|
Platelets (106/mL)
|
220.5 ± 97.1
|
203 (152 –
263.5)
|
|
Neutrophils
(106/mL)
|
6.61 ± 3.8
|
5.7 (4 – 8.5)
|
|
Lymphocytes
(106/mL)
|
1.1 ± 0.9
|
0.8 (0.6 –
1.3)
|
|
Monocytes (106/mL)
|
0.4 ± 0.3
|
0.3 (0.2 – 0.5)
|
|
NLR
|
8.2 ± 6
|
6.8 (4 –
10.7)
|
|
PLR
|
288.5 ± 206
|
228.5 (158.8 – 356.6)
|
|
MLR
|
0.5 ± 0.4
|
0.3 (0.2 – 0.6)
|
|
PT (s)
|
12.7 ± 1.1
|
12 (12 –
12.7)
|
|
PTT (s)
|
34.6 ± 0.9
|
32 (30 – 37)
|
|
BS (mg/dL)
|
157.7 ± 82.3
|
133.5 (110.1
– 170)
|
|
BUN (mg/dL)
|
22.2 ± 18.6
|
16 (11.3 – 23)
|
|
Cr (mg/dL)
|
1.24 ± 1
|
1 (0.8 – 1.2)
|
|
AST (U/L)
|
54.8 ± 44.2
|
44 (31 – 65)
|
|
ALT (U/L)
|
43.8 ± 46.6
|
29 (21 – 45.8)
|
|
ALP (U/L)
|
198 ± 85
|
178 (145.3 – 230)
|
|
LDH (U/L)
|
904 ± 372.9
|
841 (654 –
1078.8)
|
|
Alb (g/dL)
|
3.6 ± 0.5
|
3.6 (3.5 – 3.9)
|
|
ESR (mm/h)
|
54.6 ± 22.9
|
55 (39 – 66)
|
|
pH
|
7.37 ± 0.07
|
7.38 (7.34 – 7.41)
|
|
PCO2 (mmHg)
|
42.1 ± 8.3
|
41.8 (36.8 –
45.7)
|
|
HCO3 (mmol/L)
|
25.1 ± 4.1
|
25.1 (22.4 – 27.9)
|
Table
3. Rates
of disease severity, ICU admission, requiring mechanical ventilation and death
during hospitalization.
|
|
|
Frequency (Percent)
|
|
Disease severity
|
Moderate
|
160 (60%)
|
|
Severe
|
108 (40%)
|
|
ICU admission
|
No
|
190 (71%)
|
|
Yes
|
78 (29%)
|
|
Mechanical ventilation
|
Not required
|
210 (78%)
|
|
Required
|
58 (22%)
|
|
Death during hospitalization
|
No
|
205 (76%)
|
|
Yes
|
63 (24%)
|
Table
4 & 5 show the comparison of lab test results based on disease severity,
ICU admission, mechanical ventilation and death during hospitalization. White
blood cells, neutrophil count, neutrophil to lymphocyte ratio, prothrombin
time, random plasma glucose, blood urea nitrogen, AST, LDH and erythrocyte
sedimentation rate were significantly higher in patients who had severe
disease, required mechanical ventilation, were admitted to ICU or died during
hospitalization.

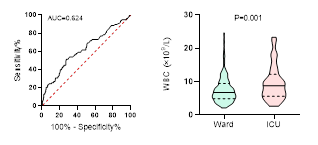
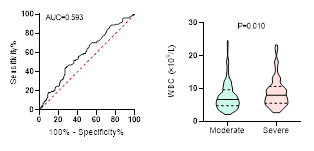
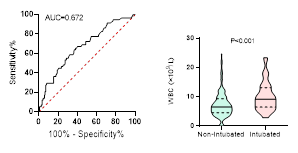
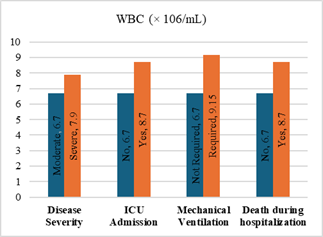
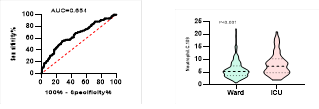
Figure
4. Predicting
potential of WBC counts on disease severity, ICU admission, requiring
mechanical ventilation and death during hospitalization.
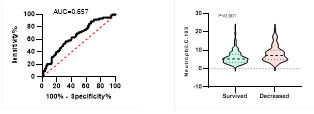
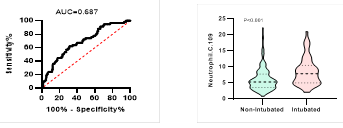
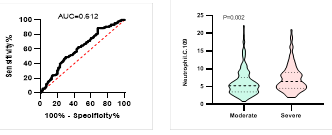
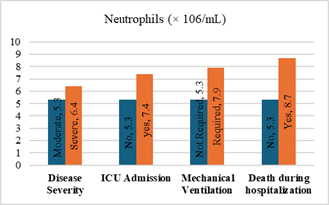
Figure
5. Predicting
potential of neutrophil counts on disease severity, ICU admission, requiring
mechanical ventilation and death during hospitalization.
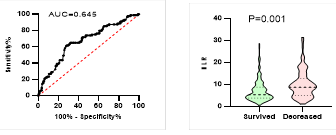
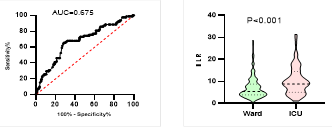
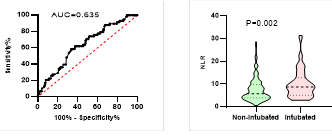
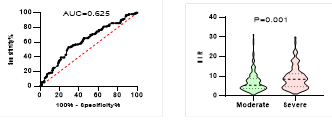
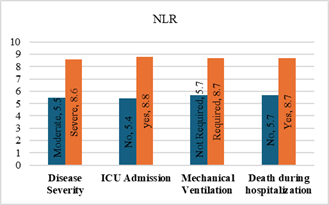
Figure
6. Predicting
potential of NLR on disease severity, ICU admission, requiring mechanical
ventilation and death during hospitalization.
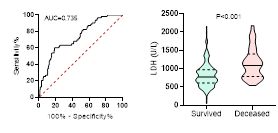
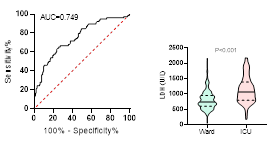
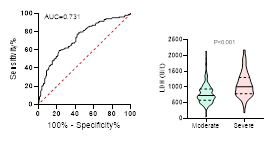
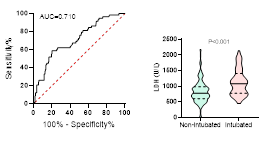
Figure
7. Predicting
potential of LDH on disease severity, ICU admission, requiring mechanical
ventilation and death during hospitalization.
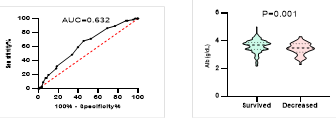
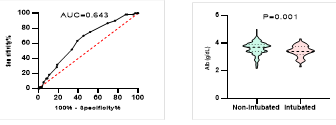
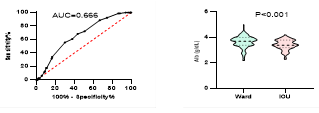
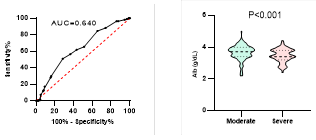
Figure
8. Predicting
potential of serum Albumin levels on disease severity, ICU admission, requiring
mechanical ventilation and death during hospitalization.
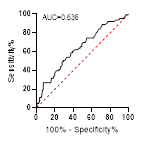
*
ROC curve is shown on the left and Violin plot is shown on the right.
Discussion
In
this study, we investigated 268 patients with mean age of 56 ± 16.6 years, of
whom 59% were female. 39% of our patients had hypertension, 28% had diabetes
mellitus, 11% had IHD, 4% had CKD and 4% had an underlying pulmonary disease.
In
our study, WBC and neutrophil count was significantly higher in patients with
severe disease, patients admitted to ICU, patients requiring mechanical
ventilation and patients who died during hospitalizations; however, our
findings didn’t show any significant difference of lymphocyte count between the
two groups of our study outcomes (in-hospital death, ICU admission, mechanical
ventilation and disease severity). An elevated neutrophil count may be an
indicator of viremia or a bacterial co-infection, which can worsen the severity
and prognosis of infected patients (10, 11). Compensatory hyperplasia of the
bone marrow which happens due to prolonged hypoxia can also result in elevated
WBC count (12). A meta analyze by Shi et al.
suggested WBC count as a mortality predictor for COVID-19 (13). Many previous studies have shown
elevated neutrophil and WBC counts, which supports our findings in this study (12, 14-21). Neutrophil
infiltration in pulmonary capillaries in autopsy studies can confirm the role
of neutrophil count in predicting disease severity and mortality (22, 23). In contrary to our findings,
numerous previous studies have shown lymphopenia as a predicting factor of
severity and different outcomes in COVID-19 (7, 16-21,
24-26). On the other
hand, Zhou et al. demonstrated that after adjusting potential risk factors,
lymphopenia didn’t have a significant effect on COVID-19 mortality (11). We only included moderate and
severe patients who met the admission criteria for COVID-19 disease in this
study. This can lead to a similar lymphocyte count in all our patients. The
mean lymphocyte counts of 1070, which indicates lymphopenia, can confirm this
hypothesis. The presence of lymphopenia in our patients is similar to previous
findings in the literature (5-7, 15,
27-30). Angiotensin converting enzyme 2, which is expressed in
lymphocytes, is the main surface receptor for SARS-CoV-2 (31); this characteristic can result in
serious damage to lymphocytes by the virus. Dramatically reduced lymphocyte
(CD8, CD4 and CD3) count can indicate the effect of virus on T-lymphocytes and
cause a major malfunction in immune system. Immunosuppression caused by
lymphocyte injury will worsen the prognosis and can cause more severe disease (7, 13).
An
elevated neutrophil count and decreased lymphocyte count results in an elevated
NLR in the patients with more severe disease and poor prognosis. The fact of
correlation between NLR and disease severity and outcome is stated by many
previous studies (12, 20, 32-34).
In
our study, LDH was significantly higher in patients with severe disease,
patients admitted to ICU, patients requiring mechanical ventilation and
patients who died during hospitalizations. The mentioned result is stated in
previous studies, too (7, 12, 17-20,
35). It is evident that LDH can be a reflecting parameter for
the extent of lung injury in ARDS, including the patients infected with
corona virus SARS (36). We also showed that ESR is
significantly higher in patients with severe disease, patients admitted to ICU,
patients requiring mechanical ventilation and patients who died during
hospitalizations. The correlation between inflammatory biomarkers including ESR
and disease outcomes is noted in a meta-analysis by Shi et al. (13).
In
our patients, serum levels of albumin were significantly lower in patients with
severe disease, patients admitted to ICU, patients requiring mechanical
ventilation and patients who died during hospitalizations. Bastug et al. also
showed lower levels of albumin in patients with more severe disease (13, 17, 33). The relevance
between the levels of serum albumin and ICU admission had been shown in MERS
infection too (37). Lower levels of serum albumin may
indicate the effect of malnutrition on disease prognosis and suggests the
benefits of nutritional support (13).
In
this study, AST levels were significantly higher in patients with severe
disease, patients admitted to ICU, patients requiring mechanical ventilation
and patients who died during hospitalizations; on the other hand, ALT levels
were only higher in patients requiring ICU admission. There wasn’t any
significant difference in alkaline phosphatase in patients with different
disease severity and outcomes. Altered liver function tests were documented in
previous studies (17); however, other studies didn’t show
any significant change in liver enzymes among different stages of disease
severity (20). There are studies showing that AST
elevates before other liver enzymes, so it can be used for patients monitoring
and predicting the disease outcome (13, 38). The direct effect of SARS-CoV-2 on
cholangiocytes is suggested as a reason of liver failure in some recent
studies. Liver injuries can occur as a result of drugs and systemic
inflammatory response, too (39). The exact reason causing liver
injuries should be investigated in further studies.
Our
findings showed longer PT and PTT in patients with severe disease, patients
admitted to ICU and patients who died during hospitalizations. PT was also
longer in patients requiring medical ventilation. Alteration in coagulation
factors is evident in previous studies (16-18); however, Wang
et al. showed that there is no difference of PT, PTT and INR among disease
severities. These results suggest that intravascular and consumption
coagulopathies can be present in COVID-19 patients with more severe disease and
hence, lead to higher mortality rates (40). Previous studies on SARS indicate
that inflammatory response may alter coagulation pathways and lead to
disseminated infarcts and hemorrhages (41).
Based
on the AUC of ROC curve we demonstrated that LDH, AST and serum albumin levels
were the most powerful predicting factors for disease severity. LDH and serum
albumin levels were also shown by Zhang et al. and HU et al. to be a potential
predicting factor for disease severity (34, 42). We showed that LDH, WBC and
neutrophil counts and NLR are significant predicting factor in ICU admission
and requirement of mechanical ventilation. Previous studies support these
results (33, 43).
Conclusion
This
study shows higher values of hematologic indices in patients with severe
disease and poor outcome. These indices can reflect inflammatory passages
(neutrophilia) and viral infection by COVID-19 (lymphopenia). Evaluated
inflammatory markers are also shown to be generally higher in patients with
poor disease outcome. The existence of coagulopathies and altered LFT in
patients with poor disease outcome can be the effect of direct viral infection
of COVID-19 and needs to be further investigated.
Limitations
We
evaluated patients with moderate and severe disease in this study. Evaluation
of patients with mild disease will give us more accurate results. Also, a
bigger sample size and a multicenter study can always help the accuracy of the
survey. Determining the predicting factors can lead to earlier treatment for
severe cases of COVID-19 and further studies to establish a cut-off for
clinical interference can be clinically beneficial.
Author
contribution
Conceptualization:
FN, AAF; Methodology: FN, BA; Formal
analysis and investigation: AMGh, SM; Writing - original draft
preparation: BA, ZCh, AMGh; Writing - review and editing: AMGh,
FN; Supervision: FN, AAF.
Acknowledgments
We declare our gratitude to all staff in Razi hospital
which devoted their lives in COVID-19 pandemia. They contributed a huge role in
gathering the information used in this study.
This
study was approved by the Ethics Committee of Guilan University of Medical
Sciences (Code: IR.GUMS.REC.1400.547, Date: 02-02-2022).
Conflict
of interest
There
is no Conflicts of interest/competing interests.
Funding
There
is no funding.
References
1. Lai C-C, et al. Severe
acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus
disease-2019 (COVID-19): The epidemic and the challenges. International journal
of antimicrobial agents. 2020;55(3):105924.
2. Gorbalenya AE, et al. Severe acute
respiratory syndrome-related coronavirus: The species and its viruses–a
statement of the Coronavirus Study Group. BioRxiv. 2020.
3. Lefkowitz EJ, et al. Virus taxonomy: the
database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic
acids research. 2018;46(D1):D708-D17.
4. Zu ZY, et al. Coronavirus disease 2019
(COVID-19): a perspective from China. Radiology. 2020;296(2):E15-E25.
5. Huang C, et al. Clinical features of
patients infected with 2019 novel coronavirus in Wuhan, China. The lancet.
2020;395(10223):497-506.
6. Chen N, et al. Epidemiological and clinical
characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan,
China: a descriptive study. The lancet. 2020;395(10223):507-13.
7. Tan L, et al. Lymphopenia predicts disease
severity of COVID-19: a descriptive and predictive study. Signal transduction
and targeted therapy. 2020;5(1):33.
8. Tang N, et al. Abnormal coagulation
parameters are associated with poor prognosis in patients with novel
coronavirus pneumonia. Journal of thrombosis and haemostasis. 2020;18(4):844-7.
9. Rahi MS, et al. Hematologic disorders
associated with COVID-19: a review. Annals of hematology. 2021;100:309-20.
10. Cervellin G, et al. Toward a holistic
approach for diagnosing sepsis in the emergency department. Advances in
clinical chemistry. 2019;92:201-16.
11. Zhou F, et al. Clinical course and risk
factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a
retrospective cohort study. The lancet. 2020;395(10229):1054-62.
12. Wang C, Deng R, Gou L, Fu Z, Zhang X, Shao F,
Wang G, Fu W, Xiao J, Ding X, Li T, Xiao X, Li C. Preliminary study to identify
severe from moderate cases of COVID-19 using combined hematology parameters.
Ann Transl Med. 2020 May;8(9):593.
13. Shi C, et al. Predictors of mortality in
patients with coronavirus disease 2019: a systematic review and meta-analysis.
BMC infectious diseases. 2021;21:1-15.
14. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y,
Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of Immune Response in
Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis.
2020;71(15):762-768.
15. Chen G, et al. Clinical and immunological
features of severe and moderate coronavirus disease 2019. The Journal of
clinical investigation. 2020;130(5):2620-9.
16. Javanian M, et al. Clinical and laboratory
findings from patients with COVID-19 pneumonia in Babol North of Iran: a
retrospective cohort study. Romanian Journal of Internal Medicine.
2020;58(3):161-7.
17. Bonetti G, et al. Laboratory predictors of
death from coronavirus disease 2019 (COVID-19) in the area of Valcamonica,
Italy. Clinical Chemistry and Laboratory Medicine (CCLM). 2020;58(7):1100-5.
18. Wang K, et al. Clinical and laboratory
predictors of in-hospital mortality in patients with coronavirus disease-2019:
a cohort study in Wuhan, China. Clinical infectious diseases.
2020;71(16):2079-88.
19. Fan BE, et al. Hematologic parameters in
patients with COVID-19 infection. American journal of hematology.
2020;95(6):E131-E4.
20. Dubey DB, et al. Hematological and serum
biochemistry parameters as a prognostic indicator of severally ill versus mild
Covid-19 patients: A study from tertiary hospital in North India. Clinical
Epidemiology and Global Health. 2021;12:100806.
21. Yu C, et al. Clinical characteristics,
associated factors, and predicting COVID-19 mortality risk: a retrospective
study in Wuhan, China. American journal of preventive medicine.
2020;59(2):168-75.
22. Fox SE, et al. Pulmonary and cardiac
pathology in African American patients with COVID-19: an autopsy series from
New Orleans. The Lancet Respiratory Medicine. 2020;8(7):681-6.
23. Yao X, et al. A pathological report of three
COVID-19 cases by minimal invasive autopsies. Zhonghua bing li xue za zhi=
Chinese journal of pathology. 2020;49(5):411-7.
24. Lippi G, Plebani M. Laboratory abnormalities
in patients with COVID-2019 infection. Clinical chemistry and laboratory
medicine (CCLM). 2020;58(7):1131-4.
25. Terpos E, et al. Hematological findings and
complications of COVID‐19. American journal of hematology. 2020;95(7):834-47.
26. Chen T, et al. Clinical characteristics of
113 deceased patients with coronavirus disease 2019: retrospective study. bmj.
2020;368.
27. Organization WH. Report of the WHO-China
joint mission on coronavirus disease 2019 (COVID-19). 2020. 2020.
28. Guan W-j, et al. Clinical characteristics of
2019 novel coronavirus infection in China. MedRxiv. 2020.
29. Shi Q, et al. Clinical characteristics of 101
non-surviving hospitalized patients with COVID-19—A single center,
retrospective study. MedRxiv. 2020:2020.03. 04.20031039.
30. Xu X-W, et al. Clinical findings in a group
of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of
Wuhan, China: retrospective case series. bmj. 2020;368.
31. Xu H, et al. High expression of ACE2 receptor
of 2019-nCoV on the epithelial cells of oral mucosa. International journal of
oral science. 2020;12(1):1-5.
32. Saurabh A, et al. Evaluation of hematological
parameters in predicting intensive care unit admission in COVID-19 patients. SN
Comprehensive Clinical Medicine. 2022;4(1):39.
33. Bastug A, et al. Clinical and laboratory
features of COVID-19: Predictors of severe prognosis. International
immunopharmacology. 2020;88:106950.
34. Zhang M, et al. An emerging marker predicting
the severity of COVID-19: Neutrophil-Lymphocyte Count Ratio. 2020.
35. Wang L. C-reactive protein levels in the
early stage of COVID-19. Medecine et maladies infectieuses. 2020;50(4):332-4.
36. Chiang C-H, et al., editors. Eight-month
prospective study of 14 patients with hospital-acquired severe acute
respiratory syndrome. Mayo Clinic Proceedings; 2004: Elsevier.
37. Saad M, et al. Clinical aspects and outcomes
of 70 patients with Middle East respiratory syndrome coronavirus infection: a
single-center experience in Saudi Arabia. International journal of infectious
diseases. 2014;29:301-6.
38. Lei F, et al. Longitudinal association
between markers of liver injury and mortality in COVID‐19 in China. Hepatology.
2020;72(2):389-98.
39. Chai X, et al. Specific ACE2 expression in
cholangiocytes may cause liver damage after 2019-nCoV infection. biorxiv.
2020:2020.02. 03.931766.
40. Favaloro EJ, Lippi G. Recommendations for
Minimal Laboratory Testing Panels in Patients with COVID-19: Potential for
Prognostic Monitoring. Semin Thromb Hemost. 2020 Apr;46(3):379-382.
41. Enjuanes L, et al. Biochemical aspects of
coronavirus replication and virus-host interaction. Annu Rev Microbiol.
2006;60:211-30.
42. Hu H, et al. Early prediction and
identification for severe patients during the pandemic of COVID-19: a severe
COVID-19 risk model constructed by multivariate logistic regression analysis.
Journal of global health. 2020;10(2).
43. Li W, et al. Early predictors for mechanical
ventilation in COVID-19 patients. Therapeutic advances in respiratory disease.
2020;14:1753466620963017.