Figure 1. Prisma Flow
Diagram.
Prescribing
clarity mapping the link between antihypertensives and breast cancer
Shadman Newaz 1*,
Moontasir Ahmed 1, Jannat Ara Tina 1, Talukder Nasif
Shahriar 1, Abdulla Bin Hridoy 1, Supritom Sarker 1,
Mohammad Zakaria Al-Aziz 1, Md. Robed Amin 2
1 Sheikh Hasina Medical College, Tangail, Bangladesh
2 Non-communicable Disease,
DGHS, Dhaka, Bangladesh
Corresponding
Authors: Shadman Newaz
*
Email: shadmannewaz11@gmail.com
Abstract
Introduction: The relationship between antihypertensive
medication and breast cancer outcomes remains a subject of growing interest in
clinical research. This systematic review aims to evaluate the potential
associations between antihypertensives and breast cancer outcomes, providing a
detailed synthesis of current evidence and identifying areas for future
research.
Methods: We conducted a systematic review of studies published between January
2014 and January 2024, in accordance with a registered protocol on the Open
Science Framework. Multiple databases were searched for English-language
studies of various designs, including clinical trials, cohort studies, and
observational studies. A total of 51 studies were selected from 1,591 records
after a rigorous screening process. The review focused on summarizing the
evidence without formal quality appraisal, adhering to the scope of this
review.
Results: Our review identified potential links between certain
antihypertensive classes, such as ACE inhibitors and calcium channel blockers,
and breast cancer outcomes. The findings indicate that specific
antihypertensive medications may influence breast cancer-specific mortality,
recurrence rates, and overall survival. The role of the Renin-Angiotensin
System and genetic predispositions emerged as important factors in these
associations. However, the review also highlights substantial evidence gaps,
particularly regarding long-term outcomes and the interaction between
antihypertensive treatment and breast cancer biology.
Conclusion: This systematic review contributes to a better understanding of the
complex relationship between antihypertensive medications and breast cancer
outcomes. Key findings suggest that healthcare providers should consider the
potential implications of specific antihypertensive drugs in patients with
breast cancer. Further large-scale randomized controlled trials with extended
follow-up are recommended to clarify these associations and inform clinical
guidelines. Our findings underscore the importance of personalized treatment
approaches and adherence to cardiovascular regimens in this patient population.
Keywords: Antihypertensive drugs, Breast Cancer Risk, Hypertension, Medication
Associations
Introduction
Ecthyma gangrenosum (EG) is a cutaneous infection Hypertension, a prevalent cardiovascular condition,
affects an estimated 1.13 billion people globally, making it one of the leading
causes of morbidity and mortality worldwide (1). Similarly, breast cancer
remains the most common malignancy among women, accounting for a significant
global health burden (2). Given the widespread use of antihypertensive
medications to manage hypertension, understanding their potential impact on
breast cancer risk has garnered increasing attention.
Recent studies have suggested potential associations
between commonly prescribed antihypertensive drugs, including
angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers
(ARBs), β-blockers (BBs), calcium channel blockers (CCBs), and diuretics, and
breast cancer development. These findings raise critical questions about how
these medications, through mechanisms such as modulation of the
renin-angiotensin system, oxidative stress, and hormonal influences, might
affect breast cancer risk and progression (3, 4). ACEIs and ARBs, for example,
may influence angiogenesis and tumor growth by altering levels of angiotensin
II, a hormone known to promote cancerous cell proliferation (5). In contrast,
β-blockers, which regulate stress hormones, have been linked to potential
protective effects against tumor progression, although evidence remains
inconclusive (6, 7).
While substantial research has focused on
well-established breast cancer risk factors, such as genetic predispositions,
hormonal influences, and lifestyle factors (8-11), the relationship between
antihypertensive drugs and breast cancer remains less clearly understood. Some
studies have indicated a possible correlation between long-term
antihypertensive use and breast cancer risk, while others have found no
significant associations (9-11). Given the complex and sometimes contradictory
findings in the literature, a comprehensive review of existing evidence is
necessary to map key concepts, evaluate current trends, and identify critical
knowledge gaps.
This systematic review adopts a scoping review
approach to provide a broad overview of the literature on the relationship
between antihypertensive medications and breast cancer outcomes. Unlike
previous systematic reviews that may have focused on specific drug classes or
mechanisms, this review seeks to encompass various study designs and outcomes
to offer a more inclusive understanding of the topic (12, 13). The objectives
are threefold: first, to map the current body of literature on the potential
links between antihypertensive drugs and breast cancer; second, to explore the
long-term effects of antihypertensive medications on breast cancer risk,
particularly given their widespread and long-term use (2); and third, to
identify evidence gaps and guide future research, ultimately shaping clinical
decision-making and public health strategies (13-15).
Methodology
Study Design and Protocol Registration
This systematic review was conducted in accordance
with a predefined protocol registered on the Open Science Framework. The review
followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses
(PRISMA) guidelines, ensuring transparency and thorough reporting of the review
process.
Inclusion and Exclusion Criteria
The review included studies published between January
2014 and January 2024 that examined the relationship between antihypertensive
medications and breast cancer outcomes. Eligible studies were of various
designs, including clinical trials, cohort studies, case-control studies, and
observational studies. Only studies published in English were considered.
Studies were included if they focused on patients diagnosed with hypertension
and explored the use of antihypertensive medications in relation to breast cancer
outcomes. Exclusion criteria included non-English studies, those without
sufficient data for extraction, study protocols, and studies addressing other
cancer types without specific reference to breast cancer and hypertension or
antihypertensive use. Studies conducted before 2014 were excluded from the
analysis.
Search Strategy
A comprehensive and refined search was conducted
across four major electronic databases: PubMed, ScienceDirect, Cochrane Central
Register of Controlled Trials (CENTRAL), and Mendeley. The search strategy
included a combination of Medical Subject Headings (MeSH) and free-text terms
designed to capture studies related to antihypertensive medications and breast
cancer outcomes. The primary concepts of the search were antihypertensive
medications, breast cancer, and hypertension.
Specific search terms included:
●
Antihypertensive classes: "angiotensin-converting enzyme inhibitors" OR "ACE
inhibitors" OR "angiotensin II receptor blockers" OR
"ARBs" OR "beta-blockers" OR "calcium channel
blockers" OR "diuretics" OR "renin-angiotensin system"
OR "antihypertensive agents."
●
Breast cancer terms: "breast cancer" OR "breast carcinoma" OR
"mammary carcinoma" OR "breast neoplasms."
●
Breast cancer subtypes: "hormone-receptor-positive" OR "HER2-positive" OR
"triple-negative breast cancer" OR "ER-positive" OR
"PR-positive."
Additionally, keywords such as "breast cancer
incidence," "breast cancer progression," "breast cancer
recurrence," "breast cancer mortality," and "breast cancer
survival" were combined with terms related to antihypertensives.
To capture a broader range of relevant studies, terms
were also expanded to include related side effects, mechanisms, and risk
assessments, such as:
●
"hypertension treatment" OR "cardiovascular drugs"
AND "breast cancer risk."
●
"antihypertensive side effects" AND "breast cancer
survival."
●
"risk of breast cancer" AND "antihypertensive
drugs."
A second search iteration focused on grey literature
sources by searching databases like Web of Science, Scopus, and Google Scholar.
Reference lists of key studies and reviews were also screened to ensure no
relevant studies were missed.
The search covered studies published from January 2014
to January 2024, and the database searches were initially performed on October
26, 2023, with an update conducted on January 26, 2024.
Screening and Data Extraction
The screening process was managed using Rayyan
software, where duplicates were removed, and studies were screened based on the
title and abstract. Two independent reviewers (MA and TS) conducted the initial
screening of studies, with disagreements resolved by a third reviewer (JT).
Full-text reviews were then conducted for studies meeting the inclusion
criteria.
Data extraction was carried out using a predesigned
Excel spreadsheet, capturing key details such as study design, patient
population, type of antihypertensive medications used, breast cancer outcomes,
and major findings. The extraction was performed by SN, with 50% of the data
verified independently by AH and SS to ensure accuracy.
Quality Appraisal
Although the primary focus of this systematic review
was to summarize and map the existing evidence rather than to critically
appraise study quality, a descriptive evaluation of study limitations and
potential biases was performed for each study. Formal quality appraisal tools,
such as the Newcastle-Ottawa Scale (for cohort and case-control studies), were
applied where appropriate, but no studies were excluded based on quality
criteria.
Data Synthesis
Due to the heterogeneity of study designs and
outcomes, a narrative synthesis was conducted. Quantitative pooling of data
(meta-analysis) was not performed due to variations in study methods,
populations, and outcome measures across the included studies. The results were
synthesized to provide a broad overview of the evidence on the relationship
between antihypertensive medications and breast cancer outcomes.
Assessment of Bias
Bias assessment was conducted using established tools
and guidelines to ensure rigorous evaluation. We employed the Cochrane Risk of
Bias tool to systematically assess the quality and risk of bias in the included
studies. This involved evaluating various aspects, such as selection bias,
performance bias, detection bias, and reporting bias. Each study was
independently reviewed by multiple researchers to ensure a consistent and
objective assessment. This methodical approach aimed to provide a comprehensive
understanding of the potential biases influencing the study outcomes and to
enhance the reliability of the systematic review’s findings.
Results
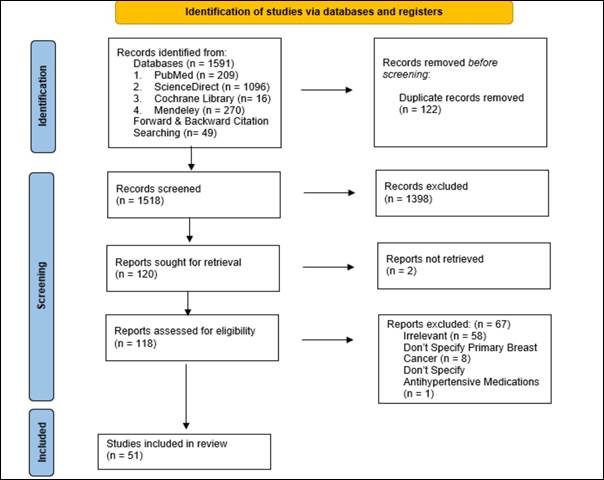
Within our study, an extensive search across key
databases, including PubMed (n = 209), ScienceDirect (n = 1096), Cochrane
Library (n = 16), and Mendeley (n = 270), yielded a total of 1,591 records.
Additionally, forward and backward citation searching contributed 49 records to
the comprehensive dataset. After removing duplications, 1,518 records underwent
meticulous screening. This process resulted in the exclusion of 1,398 records,
aligning with predefined inclusion criteria and refining the selection for
further analysis.
From the refined pool, 120 reports were sought for
retrieval, and thorough scrutiny of 118 full-texts followed. Of these, 67
full-texts were excluded based on the inclusion/exclusion criteria, as
illustrated in detail in the PRISMA flow diagram (see Fig. 1). Ultimately, our
results section will delve into the findings extracted from the inclusion of 51
unique studies (Records were consolidated when part of the same study),
offering a robust foundation for our scoping review on the intricate
relationship between antihypertensive drugs and the risk of developing breast
cancer.

Figure 1. Prisma Flow
Diagram.
Most studies were conducted in the US (n = 8) followed
by UK, China, Thailand, Taiwan having 2 studies each, and one each from
Bangladesh, South Africa, South Korea, Spain, Mexico, Israel, Australia,
Switzerland, Israel and Indonesia; 13 were multi-country studies (see Fig. 2).
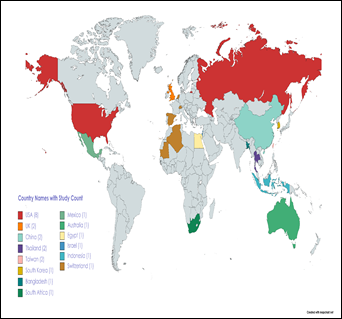
Figure 2. World Map Showing
Regions (Countries) of Included Study.
The studies varied in their methodological designs
(Table 1) which included mostly observational studies (n=30), experimental studies (n = 9), systematic
reviews with or without meta-analysis (n = 6), literature reviews (n= 4) followed
by one randomised controlled trial and one reply article. We did not perform a
quality appraisal of the included studies as our objective was to summarise the
extent and full range of evidence on the topic.
Table 1. Methodological Designs of Included Studies.
|
Study Method |
Study Count(s) |
|
Cohort/Case-control/Observation study |
30 |
|
Systematic Review & Meta-Analysis |
6 |
|
Experimental study/Animal study/In vitro study |
9 |
|
Literature Review |
4 |
|
Randomised Controlled Trial |
1 |
|
Reply Article |
1 |
Summarization of Key Findings of Each Study
Here, we present a succinct summary of the key
findings extracted from each study included in our scoping review (Table 2).
This summary captures essential insights into the nuanced relationship between
antihypertensives and breast cancer outcomes, highlighting specific
medications, genetic factors, and the role of the Renin-Angiotensin System. The
diverse array of studies contributes to a comprehensive understanding of this
complex association, informing healthcare decisions and guiding future research
endeavors (9,16-63).
Table 2. Summarization of Key Findings of Each Study.
|
Category |
Medication/Factor |
Findings |
References |
|
Calcium
Channel Blockers |
CCBs |
- Long-term use (>10 years) linked to increased breast cancer
risk. |
Supannaroj et al., 2023 (44); Stolarz et al., 2019 (34) |
|
- Mixed evidence on risk; some studies show no significant
association. |
Brasky et al., 2017 (56); Wright et al., 2017 (57) |
||
|
- Associated with specific breast cancer subtypes. |
Gómez-Acebo et al., 2016 (61) |
||
|
Beta-Blockers |
Non-Selective BBs |
- May reduce breast cancer progression and metastasis. |
Caparica et al., 2021 (17); Blaes et al., 2020 (40) |
|
- Selective BBs may increase breast cancer incidence;
non-selective BBs associated with lower recurrence risk. |
Yang et al., 2023 (43); Haldar et al., 2018 (37) |
||
|
- Promising in combination with other treatments. |
Kim et al., 2023 (38); Parada-Huerta et al., 2016 (63) |
||
|
ACE
Inhibitors and ARBs |
ACEis and ARBs |
- No consistent evidence of increased breast cancer risk. |
Chen et al., 2017 (51); Cardwell et al., 2014 (32) |
|
- Potential benefits when combined with tamoxifen. |
Ni et al., 2017 (9) |
||
|
Diuretics |
Diuretics |
- Mixed evidence; some studies suggest increased risk. |
Chen et al., 2017 (51) |
|
- Other studies find no significant impact. |
Devore et al., 2015 (28) |
||
|
β-Adrenergic
Signaling |
β-Blockers |
- Influences breast cancer progression through catecholaminergic
signaling. |
Gillis et al., 2021 (19); Busby et al., 2018 (48) |
|
- Non-selective β-blockers show efficacy in blocking tumor
growth. |
Kim et al., 2023 (36); Montoya et al., 2019 (35) |
||
|
Renin-Angiotensin
System |
RAS Inhibitors |
- Plays a significant role in breast cancer prognosis. |
Miranda et al., 2021 (18); Zhao et al., 2018 (46) |
|
- May improve clinical outcomes when combined with chemotherapy. |
Hwang et al., 2023 (45) |
||
|
Combination
Therapies |
Mixed |
- Combining antihypertensives with breast cancer treatments shows
potential but needs careful evaluation. |
Hospon et al., 2021 (20); Rico et al., 2017 (62) |
|
Adherence and
Monitoring |
Adherence |
- Non-adherence impacts blood pressure control and cancer
outcomes. |
Artignan et al., 2023 (39) |
|
- Effective management requires monitoring and adherence. |
Kozlowska et al., 2019 (47) |
||
|
Future
Research Directions |
Research Gaps |
- Gaps in understanding the impact of antihypertensive
medications on breast cancer risk and outcomes. |
Wiranata et al., 2021 (29); Han et al., 2017 (11) |
|
New Medications |
- Investigate new antihypertensive drugs and their effects on
breast cancer. |
Xia et al., 2018 (42); Kim et al., 2023 (38) |
Summarization of evidence-based
recommendations of each study
Provided below is a concise overview of evidence-based
recommendations derived from each study incorporated in our scoping review
(Table 3). This summary encapsulates key insights that offer guidance on
prescribing practices, underscore the importance of adherence to cardiovascular
drug regimens, and emphasize the need for further research to address existing
knowledge gaps. The compilation of evidence-based recommendations stems from a
diverse set of studies, enriching our understanding of the intricate interplay
between antihypertensives and breast cancer outcomes (9, 30, 31, 34, 39, 40,
49, 51, 52, 54, 55, 57).
Table 3. Summarization of evidence-based recommendations of each study.
|
Author(s) |
Recommendation |
Key Insights |
|
Leung et al., 2015 (30) |
Emphasize the need for large and comprehensive population-based
studies. |
Supports validation and further exploration of current findings. |
|
Boudreau et al., 2014 (31) |
Further evaluation of ACE inhibitors (ACEI) and beta-blockers
(BB) is needed. |
Enhances understanding of their impact on breast cancer outcomes. |
|
Stolarz et al., 2019 (34) |
Exercise caution in using calcium channel blockers (CCBs) for
breast cancer patients. |
Advises careful prescribing due to potential risks. |
|
Artignan et al., 2023 (39) |
Clinicians should be aware that non-adherence to cardiovascular
drug regimens may lead to discontinuation of adjuvant endocrine therapy
(AET). |
Highlights the link between cardiovascular and cancer treatment
adherence. |
|
Chen et al., 2017 (51) |
Most antihypertensive medications are considered safe, but
further research is needed for diuretics and β-blockers. |
Focuses on the need for safety assessment of specific
medications. |
|
Ni et al., 2017 (9) |
Conduct large, randomized controlled trials with long-term
follow-up to test the effects of certain medications on breast cancer risk. |
Calls for thorough investigation of medication impacts. |
|
Chan et al., 2022 (54) |
Investigate the long-term effects of valsartan on breast cancer
risk. |
Seeks to understand the specific implications of valsartan use. |
|
Coulson et al., 2017 (55) |
AT1R is a potential therapeutic target in breast cancer. |
Opens avenues for targeted breast cancer therapies. |
|
Wright et al., 2017 (57) |
Recommend non-randomized studies in settings with prevalent CCB
use, focusing on population-based cancer research. |
Aims to deepen insights into CCBs and breast cancer outcomes. |
Focused Summary of Recommendations
1. Validation and Further Research: Emphasize the need for large, population-based studies to validate
findings and enhance understanding of the impact of antihypertensive
medications on breast cancer outcomes (30, 31, 39).
2. Cautious Prescribing: Exercise caution with specific
antihypertensives like CCBs due to potential risks and be mindful of adherence
issues impacting cancer treatment (34, 39).
3. Safety Assessment: Continue to evaluate the safety of
diuretics and β-blockers in relation to breast cancer, and investigate the
long-term effects of specific medications such as valsartan (51, 54).
4. Therapeutic Targets: Explore AT1R as a potential therapeutic
target and conduct long-term studies to better understand medication impacts
(55, 9, 57).
Discussion
The systematic review provides a comprehensive
analysis of the relationship between antihypertensive medications and breast
cancer outcomes. This review integrates findings from various studies to
elucidate how different antihypertensive agents may influence breast cancer
risk, progression, and treatment outcomes.
Our review identifies several antihypertensive
medications that have been linked to breast cancer outcomes in varying degrees.
Notably, propranolol and atenolol have emerged as potential candidates for
further analysis due to their association with breast cancer-specific mortality
(17, 40, 62). These findings suggest that certain β-blockers might influence
disease progression differently and warrant more detailed investigation to
confirm their roles.
The role of the Renin-Angiotensin System (RAS) in
breast cancer is highlighted by studies showing its involvement in
physiological and pathological pathways that affect disease prognosis (18).
This underscores the importance of considering how antihypertensive medications
that modulate RAS might impact breast cancer outcomes.
The review also emphasizes the multifaceted role of
β-adrenergic receptor antagonists, particularly β-blockers, in influencing
breast cancer progression. These medications appear to affect cancer
progression through their action on the sympathetic nervous system, which could
open new therapeutic avenues (21). The potential for β-blockers to slow cancer
progression warrants further investigation to clarify their clinical utility.
Genetic factors, such as specific genotypes of the
AT1R A1166C SNP, are also significant. These genetic variations may contribute
to breast cancer risk, highlighting the need for personalized approaches in
treatment and risk assessment (22). Understanding these genetic influences can
help tailor therapies more effectively.
Our review brings to light several critical
recommendations for clinical practice and future research:
1. Targeted Research: The need for large, comprehensive
population-based studies is essential to validate current findings and explore
the effects of specific antihypertensive medications on breast cancer outcomes
(30, 31). Such studies could provide more robust evidence on how different
medications influence disease progression and treatment efficacy.
2. Caution in Prescription: There is a
clear need for caution when prescribing calcium channel blockers (CCBs) and
other antihypertensives in patients with breast cancer. The evidence suggests
that long-term use of these medications may be associated with increased risks,
including lymphedema and potentially adverse outcomes in breast cancer
management (34, 49). Clinical decisions should be informed by a thorough
evaluation of the risks and benefits for each patient.
3. Adherence to Cardiovascular Regimens: Ensuring adherence to cardiovascular drug regimens is crucial, as
non-adherence may lead to the discontinuation of adjuvant endocrine therapy
(AET), which is vital for breast cancer management (39). Enhancing patient
adherence through education and support can improve overall treatment outcomes.
4. Further Investigation of Specific Medications: The review highlights the need for additional
research on the safety and efficacy of diuretics and β-blockers in the context
of breast cancer (51). This includes examining their long-term effects and
interactions with other cancer treatments.
5. Exploring Genetic Factors: Genetic
variations, such as those in the AT1R A1166C SNP, should be considered in
future studies to understand their impact on breast cancer risk and treatment
(22). Incorporating genetic data could refine risk assessments and personalize
treatment strategies.
Limitations and Future Directions
This systematic review, while comprehensive, has
several limitations that must be acknowledged. First, many of the included
studies are observational in nature, which inherently limits the ability to
establish causality between antihypertensive medication use and breast cancer
outcomes. Observational studies are susceptible to various biases, such as
selection and information biases, which can affect the reliability of the
findings.
Second, potential confounding factors present a
significant challenge. Numerous studies did not adequately control for all
possible confounders, such as variations in patient demographics,
comorbidities, and concurrent treatments. This lack of control can obscure the
true relationship between antihypertensive use and breast cancer outcomes.
Third, the sample sizes in some studies were
relatively small, which may limit the generalizability of their findings. Small
sample sizes can lead to underpowered analyses, making it difficult to detect
significant associations and increasing the risk of type II errors.
Additionally, heterogeneity among studies in terms of
methodology, drug types, dosages, and follow-up periods introduces variability
in the results. This variability can complicate the synthesis of findings and
the drawing of definitive conclusions.
Finally, the review's reliance on published studies
means that it may be subject to publication bias, where studies with positive
or significant results are more likely to be published and included. This bias
can skew the overall findings of the review.
Conclusion
In conclusion, this systematic review highlights the
intricate relationship between antihypertensive medications and breast cancer
outcomes. While certain drugs like propranolol and atenolol show potential
impacts on breast cancer-specific mortality, the overall effects of
antihypertensives on breast cancer risk are complex and require further
investigation. The review emphasizes the need for large-scale, long-term
studies to clarify these relationships and improve patient management. Special
attention should be given to the risks associated with specific
antihypertensives, such as calcium channel blockers, and their interactions
with cancer therapies. Addressing these gaps will enhance treatment strategies
and patient care in this challenging area.
Author
contribution
SN led the data extraction process, developing the data charting framework
and conducting the initial charting for all included studies. SN also
contributed significantly to the analysis, interpretation of the results, and
drafting sections of the introduction and results. SN oversaw the entire review
process and coordinated the writing of the manuscript. MA was
responsible for conducting the initial search, performing the title and
abstract screening, and drafting sections of the methodology. MA also
contributed to the final review of the manuscript and played a role in
developing the study design. TS
assisted in title and abstract screening alongside MA and contributed to
refining the search strategy. TS played a key role in data extraction and
writing the methodology section of the review. JT acted as the third reviewer to resolve conflicts between MA and
TS during the screening process. JT assisted in synthesizing data and provided
feedback on the discussion and conclusion sections of the manuscript. AH verified 50% of the extracted data
to ensure accuracy and consistency. AH also reviewed the manuscript drafts and
contributed to the interpretation of study findings. SS participated in the verification of 50% of the data extraction
alongside AH and contributed to writing the discussion section. SS provided
critical revisions to the draft, focusing on improving clarity and coherence. All
authors contributed to the conception and design of the study, provided input
on the interpretation of the data, and participated in revising the manuscript.
All authors approved the final version of the manuscript before submission.
Conflict
of interest
No
conflicts of interest were reported among the authors involved in this scoping
review. All authors declare that there were no financial, personal, or
professional interests that could potentially influence the research or its
outcomes. The absence of conflicts of interest underscores the commitment to
conducting an unbiased and transparent analysis of the literature, contributing
to the credibility and reliability of the review.
Funding
There
is no funding.
References
1. Mills KT, Stefanescu A, He J. The global
epidemiology of hypertension. Nat Rev Nephrol. 2020 Apr;16(4):223-37.
2. Xie Y, Wang M, Xu P, Deng Y, Zheng Y, Yang
S, et al. Association between antihypertensive medication use and breast
cancer: A systematic review and meta-analysis. Front Pharmacol. 2021 May
13;12:609901.
3. Fan Y, Khan NH, Farhan Ali Khan M, Ahammad
MDF, Zulfiqar T, Virk R, et al. Association of hypertension and breast cancer:
Antihypertensive drugs as an effective adjunctive in breast cancer therapy.
Cancer Manag Res. 2022 Apr 1;14:1323-29.
4. Feng Y, Spezia M, Huang S, Yuan C, Zeng Z,
Zhang L, et al. Breast cancer development and progression: Risk factors, cancer
stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes
Dis. 2018 May 12;5(2):77-106.
5. Obeagu EI, Obeagu GU. Breast cancer: A
review of risk factors and diagnosis. Medicine (Baltimore). 2024 Jan 19;103(3).
6. Martin A. Genetic and hormonal risk factors
in breast cancer. J Natl Cancer Inst. 2000;92(14):1126-35.
7. Clamp A, Danson S, Clemons M. Hormonal and
genetic risk factors for breast cancer. Surgeon. 2003;1(1):23-31.
8. Shaikh F, Alamgir M, Ahmed S. Hormonal and
genetic risk factors for breast cancer in a subset of the Karachi population. J
Taibah Univ Med Sci. 2022;17(4):694-700. doi: 10.1016/j.jtumed.2021.12.006.
9. Ni H, Rui Q, Zhu X, Yu Z, Gao R, Liu H.
Antihypertensive drug use and breast cancer risk: A meta-analysis of
observational studies. Oncotarget. 2017 Jul 10;8(37):62545-60.
10. Carlos-Escalante JA, Rivas-Castro A,
Pichardo-Rojas PS, Arce C, Wegman-Ostrosky T. The use of antihypertensive drugs
as coadjuvant therapy in cancer. Front Oncol. 2021;11:660943.
11. Han H, Guo W, Shi W, Yu Y, Zhang Y, Ye X, He
J. Hypertension and breast cancer risk: A systematic review and meta-analysis.
Sci Rep. 2017;7(1):44877.
12. Pham MT, Rajić A, Greig JD, Sargeant JM,
Papadopoulos A, McEwen SA. A scoping review of scoping reviews: advancing the
approach and enhancing the consistency. Res Synth Methods. 2014
Dec;5(4):371-85.
13. Munn Z, Peters MDJ, Stern C, et al.
Systematic review or scoping review? Guidance for authors when choosing between
a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143.
14. Elmore JG, Ganschow PS, Geller BM.
Communication between patients and providers and informed decision making. J
Natl Cancer Inst Monogr. 2010;2010(41):204-9.
15. Sargeant JM, O'Connor AM. Scoping reviews,
systematic reviews, and meta-analysis: Applications in veterinary medicine.
Front Vet Sci. 2020 Jan 28;7:11.
16. Lorona NC, Cook LS, Tang MC, Hill DA, Wiggins
CL, Li CI. Antihypertensive medications and risks of recurrence and mortality
in luminal, triple-negative, and HER2-overexpressing breast cancer. Cancer
Causes Control. 2021 Dec;32(12):1375-84.
17. Caparica R, Bruzzone M, Agostinetto E, De
Angelis C, Fêde A, Ceppi M, De Azambuja E. Beta-blockers in early-stage breast
cancer: A systematic review and meta-analysis. ESMO Open. 2021;6(2):100066.
18. De Miranda FS, Guimarães JPT, Menikdiwela KR,
Mabry B, Dhakal R, Rahman RL, Moussa H, Moustaid-Moussa N. Breast cancer and
the renin-angiotensin system (RAS): Therapeutic approaches and related
metabolic diseases. Mol Cell Endocrinol. 2021;528:111245.
19. Gillis RD, Botteri E, Chang AI, Ziegler AI,
Chung NK, Pon CK, et al. Carvedilol blocks neural regulation of breast cancer
progression in vivo and is associated with reduced breast cancer mortality in
patients. Eur J Cancer. 2021;147:106-16.
20. Hopson MB, Lee S, Accordino M, Trivedi M,
Maurer M, Crew KD, et al. Phase II study of propranolol feasibility with
neoadjuvant chemotherapy in patients with newly diagnosed breast cancer. Breast
Cancer Res Treat. 2021;188(2):427-32. doi:
21. De Sanctis R, Viganò A, Torrisi R, Santoro A.
Re: Carvedilol blocks neural regulation of breast cancer progression in vivo
and is associated with reduced breast cancer mortality in patients: Sympathetic
nervous system activity on breast cancer: the story of migraine. Eur J Cancer.
2021 Jul;152:250-1.
22. El Sharkawy RM, Zaki AM, El Fattah Kamel AA,
Bedair RN, Ahmed AS. Association between the polymorphisms of angiotensin
converting enzyme (Peptidyl-Dipeptidase A) INDEL mutation (I/D) and Angiotensin
II type I receptor (A1166C) and breast cancer among postmenopausal Egyptian
females. Alex J Med. 2014;50(3):267-74.
23. Namazi S, Rostami-Yalmeh J, Sahebi E,
Jaberipour M, Razmkhah M, Hosseini A. The role of captopril and losartan in
prevention and regression of tamoxifen-induced resistance of breast cancer cell
line MCF-7: an in vitro study. Biomed Pharmacother. 2014 Jun;68(5):565-71.
24. Kang F, Ma W, Ma X, Shao Y, Yang W, Chen X,
Li L, Wang J. Propranolol inhibits glucose metabolism and 18F-FDG uptake of
breast cancer through posttranscriptional downregulation of hexokinase-2. J
Nucl Med. 2014 Mar;55(3):439-45.
25. Hugon-Rodin J, Gompel A, Plu-Bureau G.
Antihypertensive medications and breast cancer risk. JAMA Intern Med. 2014
Apr;174(4):640-1.
26. Chen L, Malone KE, Li CI. Use of
antihypertensive medications not associated with risk of contralateral breast
cancer among women diagnosed with estrogen receptor-positive invasive breast
cancer. Cancer Epidemiol Biomarkers Prev. 2015 Sep;24(9):1423-6.
27. Lamkin DM, Sung HY, Yang GS, David JM, Ma JC,
Cole SW, Sloan EK. α2-Adrenergic blockade mimics the enhancing effect of
chronic stress on breast cancer progression. Psychoneuroendocrinology. 2015
Jan;51:262-70.
28. Devore EE, Kim S, Ramin CA, Wegrzyn LR, Massa
J, Holmes MD, Michels KB, Tamimi RM, Forman JP, Schernhammer ES.
Antihypertensive medication use and incident breast cancer in women. Breast
Cancer Res Treat. 2015 Feb;150(1):219-29.
29. Wiranata S, Anjani IAW, Wulandari PA,
Indrakusuma AABP, Sadeva IGKA, Wisnawa ADF, Fajar JK, Prabawa IPY, Adiputra
PAT, Sudarsa IW, Lestari AAW, Wihandani DM, Supadmanaba IGP. The risk of
antihypertensive drugs among breast cancer patients: A systematic review and
meta-analysis. Open Access Maced J Med Sci. 2021;9(F):327-34.
30. Leung HW, Hung L, Chan ALF, Mou C. Long-term
use of antihypertensive agents and risk of breast cancer: a population-based
case–control study. Cardiol Ther. 2015;4(1):65-76.
31. Boudreau DM, Yu O, Chubak J, Wirtz HS, Bowles
EJ, Fujii M, Buist DS. Comparative safety of cardiovascular medication use and
breast cancer outcomes among women with early-stage breast cancer. Breast
Cancer Res Treat. 2014 Apr;144(2):405-16.
32. Cardwell CR, McMenamin ÚC, Hicks BM, Hughes
C, Cantwell MM, Murray LJ. Drugs affecting the renin-angiotensin system and
survival from cancer: a population-based study of breast, colorectal, and
prostate cancer patient cohorts. BMC Med. 2014 Feb 13;12:28.
33. Wilson L, D’Aloisio AA, Sandler DP, Taylor
JA. Antihypertensive use and breast cancer risk in the Sister Study. Environ
Health Perspect. 2014;122(1):2484.
34. Stolarz AJ, Lakkad M, Klimberg VS, Painter
JT. Calcium channel blockers and risk of lymphedema among breast cancer
patients: nested case-control study. Cancer Epidemiol Biomarkers Prev. 2019
Nov;28(11):1809-15.
35. Montoya A, Varela-Ramirez A, Dickerson E,
Pasquier E, Torabi A, Aguilera R, Nahleh Z, Bryan B. The beta adrenergic
receptor antagonist propranolol alters mitogenic and apoptotic signaling in
late-stage breast cancer. Biomed J. 2019 Jun;42(3):155-65.
36. Kim YJ, Jang SK, Kim G, Hong SE, Park CS,
Seong MK, Kim HA, Kim KS, Kim CH, Park KS, Hong J, Jin HO, Park IC. Nebivolol
sensitizes BT-474 breast cancer cells to FGFR inhibitors. Anticancer Res. 2023
May;43(5):1973-80.
37. Haldar R, Shaashua L, Lavon H, Lyons YA,
Zmora O, Sharon E, Birnbaum Y, Allweis T, Sood AK, Barshack I, Cole S,
Ben-Eliyahu S. Perioperative inhibition of β-adrenergic and COX2 signaling in a
clinical trial in breast cancer patients improves tumor Ki-67 expression, serum
cytokine levels, and PBMCs transcriptome. Brain Behav Immun. 2018
Oct;73:294-309.
38. Kim S, Park JM, Park S, Jung E, Ko D, Park M,
Seo J, Nam KD, Kang YK, Lee K, Farrand L, Kim YJ, Kim JY, Seo JH. Suppression
of TNBC metastasis by doxazosin, a novel dual inhibitor of c-MET/EGFR. J Exp
Clin Cancer Res. 2023 Nov 4;42(1):292
39. Artignan J, Capmas P, Panjo H, Constantinou
P, Pelletier-Fleury N. Are breast cancer patients with suboptimal adherence to
cardiovascular treatment more likely to discontinue adjuvant endocrine therapy?
Competing risk survival analysis in a nationwide cohort of postmenopausal
women. BMC Med. 2023 Nov 24;21(1):463.
40. Blaes AH, Domingo-Musibay E, Kalinsky K.
Propranolol: What is BLOCKing Its Clinical Investigation in Breast Cancer? Clin
Cancer Res. 2020 Apr 15;26(8):1781-3.
41. Ashrafi S, Shapouri R, Shirkhani A, Mahdavi
M. Anti-tumor effects of propranolol: adjuvant activity on a transplanted
murine breast cancer model. Biomed Pharmacother. 2018 Aug;104:45-51.
42. Xia T, He Q, Shi K, Wang Y, Yu Q, Zhang L,
Zhang Q, Gao H, Ma L, Liu J. Losartan-loaded liposomes improve the antitumor
efficacy of liposomal paclitaxel modified with pH-sensitive peptides by
inhibition of collagen in breast cancer. Pharm Dev Technol. 2018
Jan;23(1):13-21.
43. Yang J, Zhang S, Jiang W. Impact of beta
blockers on breast cancer incidence and prognosis. Clin Breast Cancer. 2023
Jun;23(6):664-71.e21.
44. Supannaroj R, Khamsai S, Chindaprasirt J,
Sukeepaisarnjaroen W, Limpawattana P, Sawanyawisuth K. An association between
calcium channel blocker and breast cancer in patients with hypertension: A
case-control study. Med Drug Discov. 2023;20:100168.
45. Hwang HJ, Lee TG. Impact on clinical outcomes
of renin-angiotensin system inhibitors against doxorubicin-related toxicity in
patients with breast cancer and hypertension: A nationwide cohort study in
South Korea. PLoS One. 2023 Nov 20;18(11).
46. Zhao Y, Wang Q, Zhao X, Meng H, Yu J. Effect
of antihypertensive drugs on breast cancer risk in female hypertensive
patients: Evidence from observational studies. Clin Exp Hypertens.
2018;40(1):22-27.
47. Kozłowska K, Kozłowski L, Małyszko J.
Hypertension prevalence in early breast cancer patients undergoing primary
surgery. Adv Med Sci. 2019 Mar;64(1):32-36.
48. Busby J, Mills K, Zhang SD, Liberante FG,
Cardwell CR. Postdiagnostic calcium channel blocker use and breast cancer
mortality: A population-based cohort study. Epidemiology. 2018
May;29(3):407-413.
49. Lin SY, Huang HY, Chiang LT, Huang LY, Wang
CC. Use of calcium channel blockers and risk of breast cancer among women aged
55 years and older: A nationwide population-based cohort study. Hypertens Res.
2023 Oct;46(10):2272-2279.
50. Ayeni OA, Joffe M, Mapanga W, Chen WC, O'Neil
DS, Phakathi B, Nietz S, Buccimazza I, Čačala S, Stopforth LW, Jacobson JS,
Crew KD, Neugut AI, Ramiah D, Ruff P, Cubasch H, Chirwa T, McCormack V,
Micklesfield LK, Norris SA. Multimorbidity and overall survival among women
with breast cancer: Results from the South African Breast Cancer and HIV
Outcomes Study. Breast Cancer Res. 2023 Jan 23;25(1):7.
51. Chen L, Chubak J, Boudreau DM, Barlow WE,
Weiss NS, Li CI. Use of antihypertensive medications and risk of adverse breast
cancer outcomes in a SEER-Medicare population. Cancer Epidemiol Biomarkers
Prev. 2017 Nov;26(11):1603-1610.
52. Cardwell CR, Pottegård A, Vaes E, Garmo H,
Murray LJ, Brown C, Vissers PA, O'Rorke M, Visvanathan K, Cronin-Fenton D, De
Schutter H, Lambe M, Powe DG, van Herk-Sukel MP, Gavin A, Friis S, Sharp L,
Bennett K. Propranolol and survival from breast cancer: A pooled analysis of
European breast cancer cohorts. Breast Cancer Res. 2016 Dec 1;18(1):119.
53. Chang CH, Chiang CH, Yen CJ, Wu LC, Lin JW,
Lai MS. Antihypertensive agents and the risk of breast cancer in women aged 55
years and older: A nested case-control study. J Hypertens. 2016
Mar;34(3):558-66.
54. Chan TH, Tsoi MF, Yung Cheung BM. Cancer risk
of angiotensin II receptor blocker valsartan: A population-based study. J
Cardiovasc Pharmacol. 2022 Apr 1;79(4):577-582.
55. Coulson R, Liew SH, Connelly AA, Yee NS, Deb
S, Kumar B, Vargas AC, O'Toole SA, Parslow AC, Poh A, Putoczki T, Morrow RJ,
Lazarus KA, Yeap EFW, Walton KL, Harrison CA, Hannan NJ, George AJ, Clyne CD,
Ernst M, Allen AM, Chand AL. The angiotensin receptor blocker, Losartan,
inhibits mammary tumor development and progression to invasive carcinoma.
Oncotarget. 2017 Mar 21;8(12):18640-18656.
56. Brasky TM, Krok-Schoen JL, Liu J, Chlebowski
RT, Freudenheim JL, Lavasani S, Margolis KL, Qi L, Reding KW, Shields PG, Simon
MS, Wactawski-Wende J, Wang A, Womack C, Manson JE. Use of calcium channel
blockers and breast cancer risk in the Women's Health Initiative. Cancer
Epidemiol Biomarkers Prev. 2017 Aug;26(8):1345-1348.
57. Wright CM, Moorin RE, Chowdhury EK, Stricker
BH, Reid CM, Saunders CM, Hughes JD. Calcium channel blockers and breast cancer
incidence: An updated systematic review and meta-analysis of the evidence.
Cancer Epidemiol. 2017 Oct;50:113-124.
58. Islam D, Islam MS, Jesmin. Association of
hypertension, hyperlipidemia, obesity, and demographic risk factors with breast
cancer in Bangladeshi women. Medicine (Baltimore). 2022 Nov 18;101(46).
59. Altundag K. Antihypertensive medication use
and breast cancer risk. J Hypertens. 2017 Aug;35(8):1722.
60. Schairer C, Gadalla SM, Pfeiffer RM, Moore
SC, Engels EA. Diabetes, abnormal glucose, dyslipidemia, hypertension, and risk
of inflammatory and other breast cancer. Cancer Epidemiol Biomarkers Prev. 2017
Jun;26(6):862-868.
61. Gómez-Acebo I, Dierssen-Sotos T, Palazuelos
C, Pérez-Gómez B, Lope V, Tusquets I, Alonso MH, Moreno V, Amiano P, Molina de
la Torre AJ, Barricarte A, Tardon A, Camacho A, Peiro-Perez R, Marcos-Gragera
R, Muñoz M, Michelena-Echeveste MJ, Ortega Valin L, Guevara M, Castaño-Vinyals
G, Aragonés N, Kogevinas M, Pollán M, Llorca J. The use of antihypertensive
medication and the risk of breast cancer in a case-control study in a Spanish
population: The MCC-Spain Study. PLoS One. 2016 Aug 10;11(8).
62. Rico M, Baglioni M, Bondarenko M, Laluce NC,
Rozados V, André N, Carré M, Scharovsky OG, Menacho Márquez M. Metformin and
propranolol combination prevents cancer progression and metastasis in different
breast cancer models. Oncotarget. 2017 Jan 10;8(2):2874-2889.
63. Parada-Huerta E, Alvarez-Dominguez T,
Uribe-Escamilla R, Rodriguez-Joya J, Ponce-Medrano JD, Padron-Lucio S,
Alfaro-Rodriguez A, Bandala C. Metastasis Risk Reduction Related with
Beta-Blocker Treatment in Mexican Women with Breast Cancer. Asian Pac J Cancer
Prev. 2016;17(6):2953-7.