Quercetin as a
radiosensitizer for enhanced efficacy of radiotherapy in MCF-7 breast cancer
cells
Mohammad Banparvar 1, Hamid Saeidi Saedi 2, Mona
Haddad Zahmatkesh 3 *
1 Student Research Committee, School
of Pharmacy, Guilan University of Medical Sciences, Rasht, Iran
2 Department of Radiation
Oncology, GI Cancer Screening and Prevention Research Center, School of
Medicine, Guilan University of Medical Sciences, Rasht, Iran
3 Department of Pharmaceutical
Biotechnology and Nuclear Pharmacy, School of Pharmacy, Guilan University of
Medical Sciences, Rasht, Iran
Corresponding
Authors: Mona Haddad Zahmatkesh
* Email: haddadmona629@gmail.com
Abstract
Introduction: Radiation therapy is
a primary approach for treating cancer. Utilizing natural radiosensitizer
compounds is crucial to enhance radiosensitivity in tumor tissue while
minimizing damage to normal tissue. This study aims to assess the impact of
quercetin as a radiosensitizing compound in MCF-7 cells.
Materials and Methods: This research examined the impact of quercetin at concentrations of
20, 40, and 60 μM with and without radiation (2 and 3 Gy) on the MCF-7 breast
cancer cell line as a radiosensitizer agent. The investigation employed a
micronucleus test, clonogenic assay, and assessments of Superoxide-dismutase
and catalase activity.
Results: The findings indicated that the group exposed to radiation exhibited a
significant decrease in the number of colonies (P < 0.0001) and activity of
SOD and CAT enzymes while showing a significant increase in the number of
micronuclei compared to the control group (P < 0.0001). Additionally, in all
the groups treated with quercetin and exposed to radiation, there was a notable
increase in micronuclei and a significant decrease in the number of colonies
and activity of CAT and SOD enzymes.
Conclusions: The study's findings demonstrated that quercetin has the ability to
increase the sensitivity of MCF-7 breast cancer cells to ionizing radiation in
a manner that depends on the dosage.
Keywords: Breast cancer, MCF-7, Radiosensitizing, Radiotherapy
Introduction
Cancer
is a medical condition where abnormal cells grow uncontrollably and can spread
to other parts of the body through metastasis(1). Breast cancer is a major concern,
causing a significant number of cancer-related fatalities in women globally (2). According to the World Health
Organization (WHO), cancer is one of the primary causes of death, and in 2020
alone, approximately 9.9 million people passed away due to this condition. Out
of these, 2.3 million individuals, which make up 11.7% of the total new cases,
passed away due to breast cancer, the second most commonly diagnosed type of
cancer (3). Breast cancer is commonly treated
with conventional methods such as surgery, hormone therapy, radiotherapy, and
chemotherapy (4). In advanced stages of cancer,
these treatments rarely work and often cause damage to healthy cells (5). Understanding the key factors and
molecular mechanisms of breast cancer metastasis is crucial, as it has a high
risk of relapse and can spread to vital organs like the lungs, brain, liver,
and bone, leading to fatal outcomes (6). Breast cancer prognosis has
improved due to the continuous development of medical technology. However,
recurrence and metastasis still pose major challenges (7). Moreover, drug resistance is a
common occurrence due to the high variability and compensatory adaptation
mechanisms of cancer cells, which may lead to treatment failure. Therefore, it
is crucial to develop new therapeutic strategies and drugs to treat breast
cancer effectively (8).
Radiotherapy
(RT) is a common treatment for cancer that generates reactive oxygen species in
tumor tissues, promoting apoptosis and inhibiting tumor growth (9). However, healthy tissues are
unavoidably exposed to radiation, increasing the risk of normal tissue
complications (10). To increase radiotherapy's
efficacy, radiosensitizers are used to absorb and deposit X-ray irradiation
energy in tumors (11). Studies suggest that the use of
radiosensitizing agents can improve radiotherapy treatment outcomes, leading to
better survival rates in patients with breast cancer (10). Quercetin (QUR), a natural
compound, has been extensively researched as a radiosensitizer for tumor
radiotherapy, demonstrating significant increases in tumor radiosensitivity
both in vitro and in vivo. When used systemically, quercetin is considered a
radioprotective agent (12).
Quercetin
is a flavonoid that is commonly found in many vegetables, fruits, and seeds,
such as apples, cherries, grapes, onions, broccoli, peanuts, and soybeans, as
well as beverages like tea and wine (13). Researchers have observed that it
has anticancer effects, including inhibiting cancer cell growth, invasion, and
metastasis, along with regulating autophagy, apoptosis, and immune response
enhancement (14). Studies have also shown that
quercetin can induce apoptosis and cell cycle arrest in different cancer cell
lines, such as breast, prostate, lung, and colon cancers (15). QUR is considered a promising
anticancer option because of its chemoprotective action against tumor cell
lines through metastasis and apoptosis (16). It influences the G1 phase and
induces apoptosis by suppressing cyclin D1, P21, and Twist expression in MCF-7
cells. QUR also plays an anti-proliferative role in MCF-7 cells by reducing the
phosphorylation of P38MAPK, a hallmark of cell proliferation (17).
Based
on the discovered anticancer effects of flavonoids, including quercetin, it is
hypothesized that this compound may affect breast cancer cells and their
resistance to radiation therapy, both by reducing and increasing their
sensitivity. Therefore, the present study was designed to investigate the
radiation sensitization effect of quercetin on MCF-7 breast cancer cells.
Materials and methods
Chemical,
Drug, and Reagent
Quercetin
and Cytochalasin-B were purchased from Sigma Chemicals Co. (St. Louis, USA).
SOD Assay Kit (Nasdox™–Superoxide Dismutase Assay Kit) and CAT kit (Nactaz™ -
Catalase Activity Assay Kit) were obtained from Navand Salamat Co. (Iran).
Methanol, Giemsa stain, and acetic acid were obtained from Merck (Germany).
Cell
line and cell culture
Human
breast cancer cell line MCF-7 (obtained from National Cell Bank of Tehran,
Iran) was grown in RPMI 1640 medium (Dacell, Iran) supplemented with 10% fetal
bovine serum (FBS) (Gibco), 100 units of penicillin/ml (Dacell, Iran), and 100
µg of streptomycin/ml (Dacell, Iran), incubated at 37 C in 5% CO2. The growth
medium was changed every three days and once the cells reached 80% confluence,
they were sub-cultured with 0.25% trypsin. (Gibco, UK). (18, 19).
Quercetin
treatment and ionizing radiation (IR)
At
24 h after plating the cells, the medium was removed and replaced with a fresh
medium or medium containing different concentrations of quercetin. For
treatments, cells were left untreated or treated with ionization radiation
alone or quercetin in concentrations of 20, 40, and 60 μM in 12-well plates.
For drug treatment, quercetin was added to the cultures 4 h before radiation.
The control groups were cultured without drug, with corresponding medium
amounts instead. The cells that received treatment were subjected to doses of 2
and 3 Gy of IR. The cells were irradiated with a 6 MV X-ray beam produced by a
Linear accelerator (Shinva, China) at a dose rate of 1.96 Gy/min and
source-to-sample distance (SSD) of 60 cm. Following irradiation, the plates
were transferred to the incubator at 37 ◦C under 5% CO2 and 95% humidity.
The
cytokinesis-block micronucleus (CBMN) assay
Following
the irradiation of MCF-7 cells, the culture medium that contained quercetin was
removed and replaced with a fresh medium. The plates were then placed in a CO2
incubator at 37 degrees Celsius and 95% humidity for 48 hours. "To stop
proliferation, 100 μl of cytochalasin B with a concentration of 6 μg/ml was
added to each well. The cell contents were transferred to microtubes with a 2
ml volume and a fixing solution (6:1 cold glacial acetic acid-methanol
solution) was slowly added drop by drop. Each microtube was used to prepare
three slides that were left to dry at room temperature for 24 hours before
being stained with 10% Gimsa dye for 3 minutes. The slides were washed with
distilled water for 30 seconds and dried at room temperature. Finally, the
slides were examined under a microscope (20, 21).
Clonogenic
assay
The
MCF7 cancer cells were seeded in triplicate in 6-well plates at a density of
2000 cells per well. Following an overnight incubation, the cells were
subjected to pre-determined groups and treated with or without quercetin for 3
hours. The cells were then exposed to radiation doses of 2 and 3 Gy. The cells
were incubated at 37°C in 5% CO2 and 95% humidity for 14 days. Afterward,
colonies were washed with PBS, fixed with fixative solution (methanol-acetic
acid, 6:1), and stained with 10% Giemsa (v/v) in water. Viable cells were
identified based on the presence of colonies with 50 or more cells. The plating
efficiency was calculated as follows: PE = (Number of colonies formed / Number
of cells plated) × 100%. This allows us to quantify the ability of the cells to
form colonies after treatment. The surviving fraction (SF) was determined by dividing the number
of colonies formed by the product of the number of cells plated and the plating
efficiency(22).
Superoxide
dismutase activity assay
To
evaluate Superoxide Dismutase (SOD), a Nasdox™eSuperoxide Dismutase Assay Kit
(Navand Salamat Company, Urmia, Iran) was used. After preparing a culture
medium containing at least one million cells, it was centrifuged at 800 rpm for
two minutes, and the supernatant was removed. Then, 500 μl of lysing buffer
solution was added to the cells and vortexed for 10 minutes while keeping it on
ice. The mixture was centrifuged at a speed of 12000 rpm for 5 minutes. The SOD
activity, which is considered an inhibition activity, was determined by
measuring the reduction in color development at 405 nm (23).
Catalase
activity assay
A
commercial kit Catalase (Nactaz™ Catalase Activity Assay Kit, Navand Salamat
Company, Urmia, Iran) was utilized to determine catalase (CAT) activity. 1 ml
of lysing buffer solution was used to homogenize at least 106 cells
which were then centrifuged at 8000 rpm for 10 min. The resulting supernatant
solution was used as the sample. Following 10 min of incubation at room
temperature, catalase was determined by absorbance rate at 550 nm (24).
Statistical
analysis
The results were analyzed using GraphPad Prism
software (version 7) with Two Way ANOVA - Repeated Measure. Mean ± Standard
Deviation (SD) was used to present the data, and any differences with values of
p ≤ 0.05 were considered statistically significant.
Results
Micronucleus
frequency in MCF-7 cells treated with Quercetin and radiation
The
micronucleus assay was used to assess genetic damage in MCF-7 cells treated
with quercetin and exposed to radiation. The results, illustrated in Figure 1,
demonstrate a significant increase in micronucleus frequency at quercetin
concentrations of 40 μM and 60 μM, as compared to the control group.
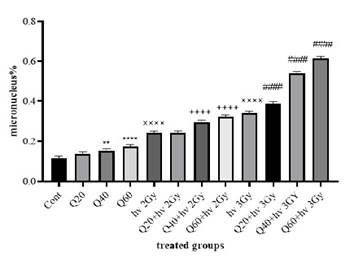
Figure
1. The
number of micronuclei in cells treated with radiation and quercetin. (Cont:
Count; Q: Quercetin; Gy: Gray; ××××:
significant difference with the control group (P < 0.0001); **: The significant difference with the control group (P <
0.01); ****: significant difference with the control
group (P < 0.0001); ++++:
the significant difference with the group receiving 2 Gy radiation (P <
0.0001); ####: the significant difference with the group
receiving 3 Gy radiation (P < 0.0001).
At a
40 μM concentration, the micronucleus frequency was significantly higher than
the control (P < 0.01), and at 60 μM, the difference was even more
pronounced (P < 0.0001). For the groups receiving radiation, the percentage
of micronuclei was 0.243% ± 0.01 for the 2 Gy radiation dose and 0.340% ± 0.01
for the 3 Gy dose, compared to 0.015% ± 0.005 in the non-irradiated control.
When
quercetin was combined with radiation, the micronucleus frequency increased
significantly. Cells treated with 40 μM and 60 μM quercetin along with 2 Gy
radiation showed a marked increase in micronuclei (P < 0.0001) compared to
cells receiving only 2 Gy radiation. Similar effects were observed with the 3
Gy radiation dose. In this case, the percentage of micronuclei in cells treated
with quercetin and 3 Gy radiation was 0.388% ± 0.01 (20 μM), 0.539% ± 0.03 (40
μM), and 0.613% ± 0.07 (60 μM), compared to 0.340% ± 0.01 in cells exposed only
to 3 Gy radiation.
Clonogenic
assay and cell survival fraction in MCF-7 cells treated with Quercetin and
radiation
The
clonogenic assay demonstrated that ionizing radiation induced cytotoxicity and
reduced cell growth (Figure 2). Both 2 Gy and 3 Gy radiation doses resulted in
a significant reduction in colony formation compared to the control group (P
< 0.0001). In the quercetin-treated groups, colony formation was also
significantly reduced, with cells treated with 40 μM and 60 μM quercetin
showing a significant decrease in the number of colonies compared to the
control (P < 0.0001).
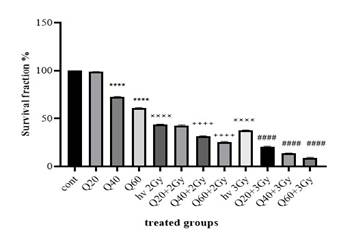
Figure
2. The
number of colonies in groups receiving quercetin, radiation, and groups without
radiation (Cont: Count; Q: Quercetin; Gy: Gray; ××××: significant difference
with the control group (P < 0.0001); ****: The significant difference with
the control group (P < 0.0001); ++++: the significant difference with the
group receiving 2 Gy radiation (P < 0.0001); ####: the significant
difference with the group receiving 3 Gy radiation (P < 0.0001)
When
quercetin was combined with radiation, a further reduction in colony number was
observed. For cells treated with 40 μM and 60 μM quercetin along with 2 Gy
radiation, colony formation was significantly lower than in the group exposed
only to 2 Gy radiation (P < 0.0001). Similarly, for cells treated with 20
μM, 40 μM, and 60 μM quercetin in combination with 3 Gy radiation, colony
numbers were significantly reduced compared to the group receiving only 3 Gy
radiation (P < 0.0001).
In
Vitro measurement of SOD activity level in MCF-7 cell treated with Quercetin
and radiation
Superoxide
dismutase (SOD) activity was measured in MCF-7 breast cancer cells treated with
quercetin and exposed to various radiation doses (Figure 3). The results showed
significant differences in SOD activity between treated groups and controls.
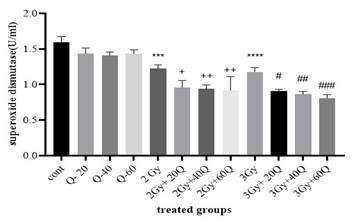
Figure
3. The
results of superoxide dismutase enzyme were measured in groups that received
quercetin, radiation, and those that did not receive radiation. (Cont: Count;
Q: Quercetin; Gy: Gray; ****: significant difference with the control group (P
< 0.0001); ++: the significant difference with the group receiving 2 Gy
radiation (P < 0.0001); ###: the significant difference with the group
receiving 3 Gy radiation (P < 0.0001); ####: the significant difference with
the group receiving 3 Gy radiation (P < 0.0001).
At
quercetin concentrations of 20, 40, and 60 μM, no significant differences in
SOD activity were observed compared to the control group. However, when cells
were treated with 40 μM and 60 μM quercetin and exposed to 2 Gy radiation, SOD
activity was significantly higher than in the group exposed to 2 Gy radiation
alone (P < 0.0001). In contrast, the 20 μM quercetin-treated group exposed
to 2 Gy radiation had significantly higher SOD activity compared to the
radiation-only group (P < 0.05).
For
cells exposed to 3 Gy radiation, SOD activity was significantly different from
the control group (P < 0.0001). Furthermore, the groups treated with 40 μM
and 60 μM quercetin followed by 3 Gy radiation exhibited significantly higher
SOD activity than the 3 Gy-only group (P < 0.001). A significant difference
was also observed between the 3 Gy radiation group and the 20 μM
quercetin-treated group (P < 0.05).
In
Vitro measurement of CAT activity level in MCF-7 cell treated with Quercetin
and radiation
Catalase
(CAT) activity was measured in MCF-7 cells treated with quercetin and exposed
to radiation. Figure 4 illustrates the enzyme activity levels across the
different experimental groups.
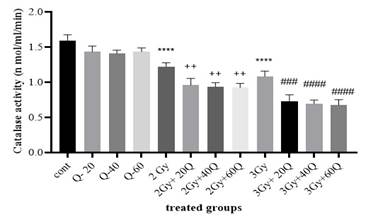
Figure
4. The
result of catalase enzyme in groups receiving quercetin, radiation, and groups
without radiation. (Cont: Count; Q: Quercetin; Gy: Gray; ***: significant
difference with the control group (P < 0.001); ****: The significant
difference with the control group; +: the significant difference with the group
receiving 2 Gy radiation (P < 0.05); ++: the significant difference with the
group receiving 2 Gy radiation (P < 0.0001); #: the significant difference
with the group receiving 3 Gy radiation (P < 0.05); ); ##: the significant
difference with the group receiving 3 Gy radiation (P < 0.01);####: the
significant difference with the group receiving 3 Gy radiation (P < 0.0001).
No
significant differences in catalase activity were observed between the
quercetin-treated groups and the control group. However, in the radiation-only
groups (2 Gy and 3 Gy), catalase activity was significantly decreased compared
to the control group (P < 0.0001).
When
quercetin was combined with radiation, a significant increase in CAT activity
was observed. Cells treated with 20, 40, and 60 μM quercetin along with 2 Gy
radiation showed significantly higher catalase activity compared to the 2
Gy-only group (P < 0.01). Additionally, cells treated with quercetin (20,
40, or 60 μM) and exposed to 3 Gy radiation exhibited significantly lower CAT
activity compared to the 3 Gy-only group (P < 0.0001). Specifically,
catalase activity in these groups was reduced to 0.731 ± 0.08, 0.695 ± 0.05,
and 0.675 ± 0.07, respectively, compared to 1.083 ± 0.07 in the 3 Gy
radiation-only group.
Discussion
Breast
cancer is still the most commonly diagnosed cancer in women. While there have
been fewer cases diagnosed in advanced, metastatic stages in recent decades, it
remains a significant public health concern globally (25). The typical treatments for breast
cancer include surgery, radiotherapy, and chemotherapy, which can all lead to
significant side effects. The goal of radiation therapy is to target the tumor
with a high dose of radiation while minimizing the impact on surrounding
healthy tissues. To address the potentially harmful effects of radiation
therapy, one approach is to use methods that make cancer cells more sensitive
to radiation. Chemotherapy drugs can help increase this sensitivity but also
come with side effects like bone marrow suppression, increased mucus
production, and skin inflammation(26). To enhance the damage caused by
radiation to cancer cells while minimizing the impact on normal tissues,
scientists have been studying substances that target cancer cells specifically
and heighten their sensitivity to ionizing radiation(27). Radiosensitizers refer to
medications or chemical compounds that amplify the lethal effects of radiation.
A reliable radiosensitizer must exhibit positive therapeutic effects, meaning
it should have a differing impact on tumors compared to normal tissues for
clinical utility(28). The mechanism behind increased
radiation sensitivity includes multiple factors such as hindering the repair of
radiation-induced damage, altering the signaling pathways of tumor cells,
initiating programmed cell death, or modifying cell metabolism(29). However, in recent years,
natural-based remedies have emerged as a potential alternative treatment option
(30). Quercetin, a powerful flavonoid
with anti-inflammatory and anti-cancer properties, can be found in various
fruits and vegetables such as citrus fruits, apples, radish leaves, and red
onions. Previous studies have shown that quercetin exhibits cytotoxic effects
on numerous types of cancer cells (31). Our study aimed to investigate the
in-vitro radiosensitizing effect of quercetin on MCF-7 breast cancer cell line.
To achieve this, we utilized the micronucleus test and colony assay to examine
the toxicity induced by quercetin on the cancer cells. We also assessed the
impact of this compound on the cells' antioxidant properties by analyzing the
activity of superoxide dismutase and catalase enzymes.
As
far as we are aware, this study is the first to explore different doses of
quercetin pretreatment in irradiated MCF-7 cell lines. Our study revealed that
pretreatment with different doses of quercetin enhances the sensitivity of
irradiated MCF-7 cell lines to radiation. This results in significantly higher
genotoxicity and reduced cell survival compared to the control groups that were
only irradiated. In the quercetin-treated groups, the number of micronuclei
increases while the number of colonies decreases. Nevertheless, the cells that
received quercetin treatment and were exposed to radiation exhibited a
significantly lower cell survival rate compared to the group that was only
exposed to radiation.
So
far, various studies have investigated the different concentrations of
quercetin potential on various cancer cells. In this regard, some have
demonstrated that Quercetin inhibited the growth, migration, and invasion and
induced apoptosis of by antagonizing SHH and TGF-β/Smad signaling pathways.
Thus, quercetin may be a potential candidate for Pancreatic ductal
adenocarcinoma treatment (32, 33). In a similar
study, researchers investigated the effect of quercetin on oxidative stress
caused by ultraviolet A (UVA) radiation in rats. Exposure to UVA rays can lead
to the production of reactive species and damage to cell components. The rats
were divided into three groups: control, exposed to UVA, and exposed to UVA and
treated with quercetin (50 mg/body weight). The results showed that the enzyme
activities of glutathione peroxidase, glutathione reductase, catalase, and
superoxide dismutase decreased significantly after irradiation. However, in the
group treated with quercetin, all of these enzyme activities were significantly
higher than in the group exposed to irradiation alone, indicating that
quercetin has a protective effect (34). Another similar study investigated
the protective effect of quercetin against oxidative stress caused by
ultraviolet radiation. Again, rats were divided into three groups: control,
ultraviolet-exposed, and ultraviolet-exposed with quercetin (50 mg/g body
weight). In the group exposed to ultraviolet radiation with quercetin, the
enzyme activity of catalase and superoxide dismutase was significantly higher
than in the group exposed to ultraviolet radiation alone, reinforcing
quercetin's potential protective effect (35). The results of the present study
showed that the activity of catalase and superoxide dismutase did not change
significantly between the control group and groups that were given different
amounts of quercetin (20, 40, and 60 μM). However, radiation increased the
level of reactive species in cells and depleted the storage of antioxidant
enzymes (catalase and superoxide dismutase). The study discovered that the
activity of catalase and superoxide dismutase was significantly different in
the group that was exposed to 2 and 3 Gy radiation compared to the control
group. This suggests that the radiation caused more oxidative stress. In
contrast to previous research, the groups that were given quercetin and then
exposed to radiation (2 and 3 Gy) had significantly different levels of the
enzymes catalase and superoxide dismutase compared to the group that was only
exposed to radiation. The levels of these enzymes were lower in the groups that
were given quercetin and then radiation. This suggests that quercetin may act
differently in cancer cells compared to normal tissue, causing a reduction in
these vital enzymes in cancer cells.
In a
similar study, the result showed that 40 μM quercetin significantly reduced the
number of MCF-7 cells (36). Additionally, Li et al.
demonstrated in 2018 that quercetin at 50 IC50 μM experimentally reduced the
survival rate of MCF-7 cells (37). Also, Niazvand et al.'s study
found that solid lipid nanoparticles containing 25 μmol of quercetin lowered
the number of MCF-7 cells by stopping their growth and killing them (38). In our study, we saw that
quercetin at 40 and 60 μM greatly increased the number of micronuclei compared
to the control group, which was made up of MCF-7 cells that had not been
treated with quercetin. An increase in the number of micronuclei indicates
damage to the DNA molecule, which ultimately leads to cell death. Also,
counting the colonies showed that the number of colonies was much lower in the
group that was given 40 and 60 μM quercetin compared to the control group. This
results confirms that the survival rate of cells treated with quercetin has
decreased.
In a
different study, researchers investigated the effects of quercetin on the
cellular response to ionizing radiation in the HepG2 cell line. They used gamma
rays at 1, 5, and 10 Gy, along with quercetin at concentrations of 10, 20, 40,
80, and 100 μM. The findings showed that cell survival decreased after a
24-hour treatment with quercetin. Additionally, the cell survival rate was
significantly lower in the group treated with both quercetin and ionizing
radiation compared to the group treated with quercetin alone. The combined
treatment of quercetin and ionizing radiation also reduced the activity of
catalase and superoxide dismutase. These results suggested that combining
quercetin with ionizing radiation could enhance the efficacy of radiation
therapy (39). In our study, like the research
mentioned above, quercetin increased the effect of ionizing radiation on the
studied cells (MCF-7), which was associated with an increase in the number of
micronuclei and a decrease in colonies. Ionizing radiation causes DNA damage
through the generation of active species and direct effects, ultimately
resulting in cell death. This genetic damage leads to an increase in the number
of micronuclei, indicating cellular damage. On the contrary, radiation caused a
greater reduction in the activity of two enzymes, catalase and superoxide
dismutase, in the groups treated with both quercetin and radiation compared to
the group treated with radiation alone. The decline in the activity of these
two antioxidant enzymes was attributed to the oxidative stress induced by
quercetin and ionizing radiation on cancer cells. In addition, quercetin has
been shown to enhance the effects of radiation in MDA-MB-231 breast cancer
cells (36), which further supports the idea that quercetin's radiosensitizing
effects may be generalizable across different breast cancer subtypes. Similar
to our findings, other studies have shown that quercetin reduces cell survival
and increases DNA damage when combined with radiation, suggesting that
quercetin may play a role in preventing DNA repair in cancer cells, thereby
amplifying radiation-induced cell death.
Research
conducted by Lin et al. in 2008 demonstrated that the presence of 40 micromoles
of quercetin significantly decreased the number of MCF-7 cells (40). Additionally,
in 2018, Li et al. revealed that quercetin with an IC50 of 50 micromoles
effectively reduced the viability of MCF-7 cells(41). In our
investigation, we found that quercetin at concentrations of 40 and 60 μM led to
a noticeable increase in the quantity of micronuclei, differing significantly
from the control group (MFC-7 cells that weren't treated with quercetin). An
upsurge in micronuclei is indicative of DNA damage, which could ultimately
result in cell death. Furthermore, the assessment of colony quantity
illustrated that in the 40 and 60 micromolar quercetin-treated groups, the
number of colonies was markedly lower than in the control group, reaffirming
the decrease in cell survival rate following quercetin treatment.
In
2022, Askar and colleagues conducted a study exploring the impact of combining
quercetin nanoparticles with targeted radiation therapy for treating breast
cancer. in vitro research demonstrated that incubating MCF-7, Hepg-2, and A459
cancer cells with quercetin nanoparticles for 24 hours resulted in the
inhibition of cancer cell growth. Furthermore, the combination of quercetin
nanoparticle treatment with radiation therapy effectively suppressed the
proliferation of MCF-7 cancer cells. During the in vivo phase, female albino
mice with breast cancer exhibited inhibited tumor growth and significantly
enhanced response to radiotherapy when treated with quercetin nanoparticles.
Consequently, the study concluded that the combination of quercetin
nanoparticles and radiation therapy could serve as an effective treatment
approach for controlling and treating breast cancer(10). Our study findings were in line
with Askar et al.'s research. We observed a significant increase in the number
of micronuclei in the quercetin group treated with radiation compared to the
radiation-only group. An elevated number of micronuclei indicates damage to the
cell's genetic material. Additionally, we conducted colony counting alongside
the micronucleus assay to further elucidate quercetin's effect. A comparison of
the number of colonies in the two aforementioned groups revealed that quercetin
caused a more pronounced reduction in colony count. Consequently, the survival
rate of the breast cancer cell line (MCF-7) in the group treated with quercetin
and radiation was lower than that in the radiation-only group.
Comparing
our results with those of other well-established radiosensitizers, such as
cisplatin and gemcitabine, also reveals interesting insights. Both cisplatin
and gemcitabine have been
extensively
studied for their ability to enhance the effects of radiation, and their
mechanisms of action include interference with DNA repair and cell cycle
progression (42). Quercetin shares some of these mechanisms, including the
inhibition of antioxidant enzymes and the induction of oxidative stress (34,
35), which may contribute to its radiosensitizing effects. However, unlike
cisplatin and gemcitabine, which are cytotoxic to both cancer and normal cells,
quercetin appears to be more selective, potentially causing less toxicity to
normal tissues (28). This selective toxicity could make quercetin a promising
candidate for further development as a radiosensitizer, especially when
combined with targeted radiation therapy, as demonstrated by Askar et al. (10),
who found that quercetin nanoparticles enhanced the radiosensitivity of MCF-7
cells both in vitro and in vivo.
In
contrast to previous studies on quercetin’s effects in normal cells, our
results suggest that quercetin may cause a reduction in antioxidant enzyme
levels specifically in cancer cells, which may enhance the radiosensitizing
effects of radiation in tumor cells. These findings suggest that quercetin’s
impact on antioxidant defense systems is context-dependent, acting differently
in cancer cells compared to normal tissues, and may contribute to its selective
radiosensitizing properties.
Conclusion
In
our current research, we examined the impact of quercetin on the MCF-7 breast
cancer cell line under both non-radiated and radiated conditions. Overall, the
findings of this study revealed that exposure to ionizing radiation leads to
cellular damage and decreased survival rates in breast cancer cell lines,
resulting in elevated micronuclei levels and reduced colony numbers compared to
the control group. Treatment with quercetin produced similar outcomes. However,
in cells treated with quercetin and exposed to radiation, the cell survival
rate was notably lower than in the group subjected solely to radiation.
Moreover, the levels of superoxide dismutase and catalase enzymes in
quercetin-treated cells exposed to radiation quercetin were significantly lower
than those in the group subjected only to radiation.
While
the findings of this study suggest that quercetin can act as a potent
radiosensitizer in MCF-7 breast cancer cells, there are several limitations
that need to be acknowledged. First, the current study was performed
exclusively in vitro using the MCF-7 cell line, which represents only one
subtype of breast cancer. The results may not be fully representative of the
diverse molecular and genetic characteristics present in other breast cancer
subtypes or in tumors from different patients. Therefore, additional studies
utilizing a broader range of breast cancer cell lines, including
triple-negative breast cancer (TNBC) or HER2-positive subtypes, would provide a
more comprehensive understanding of quercetin's radiosensitizing potential
across different cancer types.
Second,
although we observed significant effects of quercetin on cell survival and
genotoxicity, the underlying molecular mechanisms responsible for its
radiosensitizing effects need further exploration. For example, while our study
focused on the alteration of antioxidant enzyme activity, it would be valuable
to investigate the effects of quercetin on other key signaling pathways
involved in DNA damage repair, cell cycle regulation, and apoptosis.
The
next logical step would be to investigate quercetin’s radiosensitizing effects
in in vivo models, where tumor growth, metabolism, and drug bioavailability can
be better assessed in the context of the entire organism. Animal studies,
particularly in mouse xenograft models of breast cancer, would provide a more
accurate reflection of how quercetin interacts with radiation in a living
system, including its pharmacokinetics and potential toxicity.
Acknowledgments
We
extend our appreciation to the Deputy of Research and Technology at Guilan
University of Medical Sciences for their valuable assistance and consultations
during the course of this research.
Ethical
approval
All
the experimental procedures in this study were approved by the Ethics Committee
of Guilan University of Medical Sciences, Rasht, Iran (ethical code
IR.GUMS.REC.1402.619).
Author
contribution
MHZ Conceptualization, editing, review, and supervision. MB Written
and Laboratory tests. HSS Radiation therapy.
Conflict
of interest
There
is no Conflicts of interest/competing interests.
Funding
There
is no funding.
References
1. Dongsar TT, et al.
Emerging application of magnetic nanoparticles for breast cancer therapy.
European Polymer Journal. 2023;187:111898.
2. Tiwari P, et al. Dacarbazine-primed carbon
quantum dots coated with breast cancer cell-derived exosomes for improved
breast cancer therapy. Journal of Controlled Release. 2024;365:43-59.
3. Debnath J, et al. A comparative study of
diaryl urea molecules with and without sulfonamide group on Carbonic anhydrase
IX and XII inhibition and its consequence on breast cancer cells. Bioorganic
Chemistry. 2024:107192.
4. Maghsoudian S, et al. Targeted pH- and
redox-responsive AuS/micelles with low CMC for highly efficient sonodynamic
therapy of metastatic breast cancer. Biomaterials Advances. 2024;158:213771.
5. Elbeltagi S, et al. Biosynthesis,
characterization, magnetic hyperthermia, and in vitro toxicity evaluation of
quercetin-loaded magnetoliposome lipid bilayer hybrid system on MCF-7 breast
cancer. Biochimica et Biophysica Acta (BBA) - General Subjects. 2024;1868(3):130543.
6. Ismail A, et al. Beneficial and detrimental
aspects of miRNAs as chief players in breast cancer: A comprehensive review.
International Journal of Biological Macromolecules. 2023;224:1541-65.
7. Guo J, et al. Artificial intelligence:
opportunities and challenges in the clinical applications of triple-negative
breast cancer. British Journal of Cancer. 2023:1-9.
8. Luo K-f, et al. Molecular mechanisms and
therapeutic applications of huaier in breast cancer treatment. Frontiers in
Pharmacology. 2024;14:1269096.
9. Huang C, et al. Enhanced Tumor Targeting
and Radiotherapy by Quercetin Loaded Biomimetic Nanoparticles. Frontiers in
Chemistry. 2020;8.
10. Askar MA, et al. Synergistic Effect of
Quercetin Magnetite Nanoparticles and Targeted Radiotherapy in Treatment of
Breast Cancer. Breast Cancer: Basic and Clinical Research.
2022;16:11782234221086728.
11. Ma T, et al. Quercetin-Modified Metal–Organic
Frameworks for Dual Sensitization of Radiotherapy in Tumor Tissues by
Inhibiting the Carbonic Anhydrase IX. ACS Nano. 2019;13(4):4209-19.
12. Jiang W, et al. CuS@ MOF-based well-designed
quercetin delivery system for chemo–photothermal therapy. ACS applied materials
& interfaces. 2018;10(40):34513-23.
13. Farag MR, et al. Quercetin Alleviates the
Immunotoxic Impact Mediated by Oxidative Stress and Inflammation Induced by
Doxorubicin Exposure in Rats. Antioxidants [Internet]. 2021; 10(12).
14. Ding L, et al. Quercetin induces ferroptosis
in gastric cancer cells by targeting SLC1A5 and regulating the p-Camk2/p-DRP1
and NRF2/GPX4 Axes. Free Radical Biology and Medicine. 2024;213:150-63.
15. Aghababaei F, Hadidi M. Recent Advances in
Potential Health Benefits of Quercetin. Pharmaceuticals. 2023;16(7):1020.
16. Shorobi FM, et al. Quercetin: A Functional
Food-Flavonoid Incredibly Attenuates Emerging and Re-Emerging Viral Infections
through Immunomodulatory Actions. Molecules. 2023;28(3):938.
17. Ezzati M, et al. A review on anti-cancer
properties of Quercetin in breast cancer. Life Sciences. 2020;248:117463.
18. Asano S, et al. Blockade of vasoactive
intestinal peptide receptor 2 (VIPR2) signaling suppresses cyclin D1-dependent
cell-cycle progression in MCF-7 cells. Journal of Pharmacological
Sciences. 2024;154(3):139-47.
19. Comşa Ş, et al. The story of MCF-7 breast
cancer cell line: 40 years of experience in research. Anticancer research.
2015;35(6):3147-54.
20. Sommer S, et al. Micronucleus Assay: The
State of Art, and Future Directions. International Journal of Molecular
Sciences. 2020;21(4):1534.
21. Thomas P, Fenech M. Cytokinesis-Block
Micronucleus Cytome Assay in Lymphocytes. In: Didenko VV, editor. DNA Damage
Detection In Situ, Ex Vivo, and In Vivo: Methods and Protocols. Totowa, NJ:
Humana Press; 2011. p. 217-34.
22. Raeisi F, et al. Bromelain Inhibitory Effect
on Colony Formation: An In vitro Study on Human AGS, PC3, and MCF7 Cancer
Cells. J Med Signals Sens. 2019;9(4):267-73.
23. Samarghandian S, et al. Oxidative stress and
apoptotic index modifications in the hippocampus of rat pups born to mothers
exposed to buprenorphine during lactation. Toxicology Reports. 2022;9:2050-4.
24. Izanloo H, et al. The effects of varying
concentrations of glutathione and trehalose in improving microscopic and
oxidative stress parameters in Turkey semen during liquid storage at 5 °C.
Cryobiology. 2021;101:12-9.
25. Filip CI, et al. Breast Cancer Screening and
Prophylactic Mastectomy for High-Risk Women in Romania. Medicina.
2024;60(4):570.
26. Van Cutsem E, et al. Towards a pan-European
consensus on the treatment of patients with colorectal liver metastases.
European journal of cancer (Oxford, England : 1990). 2006;42(14):2212-21.
27. Linam J, Yang LX. Recent developments in
radiosensitization. Anticancer Res. 2015;35(5):2479-85.
28. Snider JW, 3rd, Mehta M. Principles of
radiation therapy. Handbook of clinical neurology. 2016;134:131-47.
29. Araujo LH, et al. 69 - Cancer of the Lung:
Non–Small Cell Lung Cancer and Small Cell Lung Cancer. In: Niederhuber JE,
Armitage JO, Kastan MB, Doroshow JH, Tepper JE, editors. Abeloff's Clinical
Oncology (Sixth Edition). Philadelphia: Elsevier; 2020. p. 1108-58.e16.
30. Hatami M, et al. Effective inhibition of
breast cancer stem cell properties by quercetin-loaded solid lipid
nanoparticles via reduction of Smad2/Smad3 phosphorylation and β-catenin
signaling pathway in triple-negative breast cancer. Biochemical and Biophysical
Research Communications. 2023;664:69-76.
31. Hatami M, et al. Quercetin-loaded solid lipid
nanoparticles exhibit antitumor activity and suppress the proliferation of
triple-negative MDA-MB 231 breast cancer cells: implications for invasive
breast cancer treatment. Molecular Biology Reports. 2023;50(11):9417-30.
32. Feng J, et al. Quercetin restrains
TGF-β1-induced epithelial–mesenchymal transition by inhibiting Twist1 and
regulating E-cadherin expression. Biochemical and biophysical research
communications. 2018;498(1):132-8.
33. Guo Y, et al. Quercetin suppresses pancreatic
ductal adenocarcinoma progression via inhibition of SHH and TGF-β/Smad
signaling pathways. Cell Biology and Toxicology. 2021;37:479-96.
34. Erden Inal M, et al. Beneficial effects of
quercetin on oxidative stress induced by ultraviolet A. Clinical and
experimental dermatology. 2001;26(6):536-9.
35. Inal ME, Kahraman A. The protective effect of
flavonol quercetin against ultraviolet a induced oxidative stress in rats.
Toxicology. 2000;154(1-3):21-9.
36. Lin C-W, et al. Quercetin inhibition of tumor
invasion via suppressing PKCδ/ERK/AP-1-dependent matrix metalloproteinase-9
activation in breast carcinoma cells. Carcinogenesis. 2008;29(9):1807-15.
37. Li X, et al. Quercetin suppresses breast
cancer stem cells (CD44+/CD24−) by inhibiting the PI3K/Akt/mTOR-signaling
pathway. Life sciences. 2018;196:56-62.
38. Niazvand F, et al. Effects of
quercetin-loaded nanoparticles on MCF-7 human breast cancer cells. Medicina.
2019;55(4):114.
39. Jang J, et al. Effects of quercetin on
ionizing radiation-induced cellular responses in HepG2 cells. International
Journal of Radiation Research. 2017;15(3):229-39.
40. Lin CW, et al. Quercetin inhibition of tumor
invasion via suppressing PKC delta/ERK/AP-1-dependent matrix
metalloproteinase-9 activation in breast carcinoma cells. Carcinogenesis.
2008;29(9):1807-15.
41. Li X, et al. Quercetin suppresses breast
cancer stem cells (CD44(+)/CD24(-)) by inhibiting the PI3K/Akt/mTOR-signaling
pathway. Life Sci. 2018;196:56-62.