A rare case of bronchial glomus tumour
of uncertain malignant potential
Sharanya
Sathish 1, Shanthi Velusamy 2 *, Divya Vijayanarasimha 2,
Arjun S Kashyap 1, Sanjeev Kulkarni 3
1 Sri Shankara Cancer Hospital and Research Centre, Bengaluru, India
2 Department of Pathology, Sri Shankara Cancer Hospital and Research
Centre, Bengaluru, India
3 Department of Surgical Oncology, Sri Shankara Cancer Hospital and
Research Centre, Bengaluru, India
Corresponding
Authors: Shanthi Velusamy
* Email: shanz84@gmail.com
Abstract
Introduction: Glomus tumours are typically benign pericytic
mesenchymal neoplasms, occurring in the soft tissues of distal extremities, and
rarely seen in deep soft tissues and visceral locations. Malignant glomus
tumours are exceedingly uncommon. Here, we present a rare case of glomus tumour
with uncertain malignant potential, arising in the bronchus of a young female
patient. This case report highlights the clinical, radiological, and
pathological aspects of this unusual entity, discussing the challenges in
diagnosis and management and reviewing relevant literature on its behaviour and
treatment options.
Case Presentation: A 35-year-old lady with
progressive breathlessness and cough, was found to have an obstructive mass
lesion in the right upper lobe bronchus on CT scan. Check bronchoscopy and
biopsy revealed features of a low-grade spindle cell lesion, with the possible
differentials of a Glomus tumour and low-grade myofibroblastic tumour. She
underwent right upper lobectomy; the final histopathological exam with the aid
of immunohistochemistry confirmed the diagnosis of a Glomus tumour of uncertain
malignant potential. Post-operatively, due to localised disease with negative
surgical margins, she was kept on regular follow-up and was asymptomatic 6
months post-surgery.
Discussion: The possibility of a Glomus tumour must be considered for tumours with
plump spindle to round cell morphology on a bronchoscopic biopsy.
Immunohistochemistry helps to exclude most differentials. The assessment of
malignant potential in Glomus tumour requires thorough examination of a
completely resected tumour sample. Molecular studies have shown NOTCH1 gene
rearrangements in both benign and malignant Glomus tumours. However, it does
not help to predict malignant potential in benign-appearing tumours.
Conclusion: Bronchial Glomus tumours of uncertain malignant potential are rare
tumours requiring more research on molecular markers for prognostication and
treatment. This case is presented for its rarity, diagnostic and prognostic
challenges.
Keywords: Glomangiomyoma, Coin lesion, Glomus Tumour of Uncertain Malignant
Potential, SMA, Glomus classification
Introduction
Glomus tumours (GT) originate from the glomus cells of
the glomus body, a neuro-myo-arterial structure involved in regulating
cutaneous circulation. These tumours constitute less than 2% of soft tissue
tumours (1) and usually arise in distal extremities. Rare sites of
occurrence include the gastrointestinal tract, genitourinary system,
mediastinum, nerves, bones, and lungs (2). When arising in the bronchopulmonary tract, a number of entities enter
the differential diagnosis and the possibility of glomus tumour may not be
suspected, due to its rarity. The differentials considered in this site usually
include carcinoid tumour, solitary fibrous tumour, sclerosing haemangioma,
leiomyoma, paraganglioma etc. Though surgery remains the primary modality
of treatment for most of these tumours, the prognosis, behaviour and adjuvant
treatments vary and therein lies the importance of accurate diagnosis. Glomus
tumours have been conventionally classified as benign, ‘of uncertain malignant
potential’ and malignant. The assessment of malignant potential of GT is
challenging on small bronchoscopic biopsies as they may not represent the
entire picture. This is especially important for malignant Glomus tumours, as
they behave very aggressively. This case report discusses a rare case of a
young female with an endobronchial glomangiomyoma of uncertain malignant
potential. The clinical and diagnostic work-up, treatment given and relevant
published literature are discussed.
Case
presentation
A 35-year-old lady presented
with progressively worsening intermittent cough and breathlessness on exertion
over a period of 1 month. She did not have fever, loss of appetite, weight
loss, or pain. Her medical history was unremarkable. A chest CT scan (Figure 1)
revealed an exo-endoluminal mass lesion occluding the right main stem bronchus;
the endoluminal component appeared hypodense and exoluminal component appeared
hyperdense with intrabronchial extension at places. Check bronchoscopy
confirmed a mass lesion in the right main bronchus causing near-complete
occlusion. Tumour debulking was performed in view of obstruction, using
electrocautery snare under general anaesthesia. Cryo-debulking was also
performed. The tumour was noted to be arising from the right upper lobe
bronchus with the rest of the right bronchial tree being normal. Argon plasma
coagulation was used to control bleeding after debulking. The biopsy sample was
subjected to histopathological evaluation and immunohistochemistry. At this
point of time, the provisional clinical diagnosis of neuroendocrine tumour was
considered.
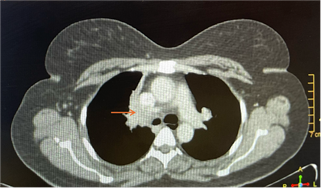
Figure 1. CT thorax
showing right bronchial hypodense endoluminal mass (arrow).
Histologically, the biopsy
showed a neoplasm composed of spindle to oval cells with pale eosinophilic
cytoplasm and plump ovoid to spindle nuclei, arranged in sheets and fascicles.
There was no significant nuclear atypia; mitotic figures were sparse (less than
1 per 2 square mm). Thin-walled branching blood vessels were present without evidence
of calcifications or necrosis. Immunohistochemical staining demonstrated strong
tumour positivity for Vimentin, alpha-SMA (Smooth Muscle Actin), focal S100
positivity, and a Ki67 index of ~12% at hotspots. Negative stains included
PanCK (excluding a salivary neoplasm), neuroendocrine markers -Synaptophysin
& Chromogranin (excluding carcinoid), STAT6 (excluding solitary fibrous
tumour), Desmin (excluding leiomyoma), TLE1 (excluding synovial sarcoma) and
ALK (for inflammatory myofibroblastic tumour). A brisk inflammatory activity
characteristic for inflammatory myofibroblastic tumour (IMT) was not evident in
the limited sampling. The differentials considered at this stage were a Glomus
tumour and a low-grade myofibroblastic tumour, in view of spindled morphology,
SMA positivity and relatively low Ki67 index. The final diagnosis was reserved
for the resection specimen to evaluate other areas of the tumour.
A PET-CT scan confirmed
localized disease with low grade FDG uptake (SUV max 3.5). In view of features
suspicious for a localised neoplastic pathology of uncertain malignant
potential, the patient subsequently underwent right upper lobectomy. The
resected specimen (Figure 2) showed a solid, grey-white endobronchial tumour
involving the largest and another branching bronchus, measuring 3.5x1.5x1.5 cm.
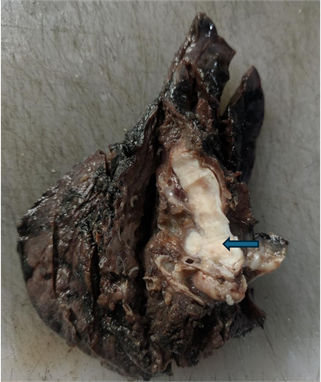
Figure 2. Lobectomy
specimen with cross section of main bronchus displaying grey-white tumour
filling the lumen (arrow).
The microscopic examination
revealed a tumour composed of oval to spindle cells arranged in sheets with
interspersed thin-walled blood vessels and separated by focal hyalinized stroma
(Figure 3A). A few areas resembling smooth muscle fibres in fascicles were also
observed (Figure 3B).
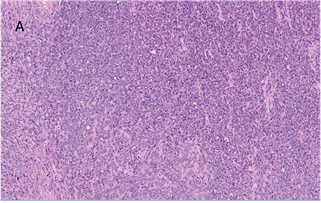
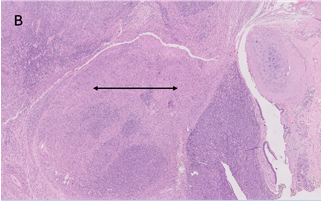
Figure 3. A. Microscopic
examination shows sheets of spindled cells with scant hyalinised matrix
(H&E, 100X). B. Smooth muscle component seen at places within the tumour
(arrow) (H&E, 40X).
The tumour cells exhibited
scant cytoplasm, elongated spindled to oval nuclei, vesicular chromatin,
occasional intranuclear grooves, and inconspicuous nucleoli (Figure 4).
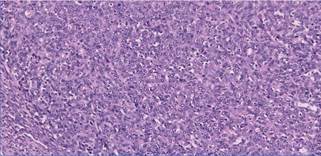
Figure 4. Microscopic
examination-high power magnification shows spindled tumour cells with plump
spindle to oval nuclei, vesicular chromatin, and inconspicuous nucleoli.
(H&E, 400X).
Some areas showed moderate
nuclear atypia. The mitotic rate was 3 per 2 sq. mm. Lymphovascular and
perineural invasion were not identified. Seven perihilar lymph nodes showed
reactive hyperplasia. Immunohistochemical analysis of the resected tumour showed
similar findings as seen in the bronchoscopic biopsy, including strong
positivity for vimentin and SMA (Figure 5). h-Caldesmon was positive only in
the myomatous component and negative in other areas. There was no inflammatory
component to suggest inflammatory myofibroblastic tumour (IMT).
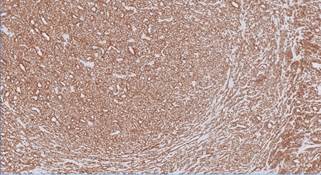
Figure 5. Diffuse strong
cytoplasmic staining for smooth muscle actin (SMA) seen in tumour cells by
IHC.
The characteristic
perivascular concentric arrangement of tumour cells seen in myopericytoma and
biphasic zonation phenomenon (central spindle cells with hemangiopericytomatous
pattern and peripheral hyalinised area) of a myofibroma were not seen; both conditions
are generally seen in dermis and subcutis and very rarely in deep locations.
The complete histological and immunohistochemical evaluation of the tumour
showed morphology consistent with a Glomangiomyoma. Invasion into the bronchial
wall and peribronchial fibrous tissue was observed focally (Figure 6). There
was no evidence of marked nuclear atypia or atypical mitosis to indicate
malignancy. Applying WHO classification of Glomus tumours (2), due to focal
moderate nuclear atypia, infiltration of the bronchial wall, deep location, and
size exceeding 2 cm, the diagnosis of Glomus tumour (Glomangiomyoma) of
uncertain malignant potential (GTUMP) was made. In view of negative surgical
margins and absence of metastatic disease, the patient was placed on close
follow-up and remained asymptomatic at 6 months post-surgery.
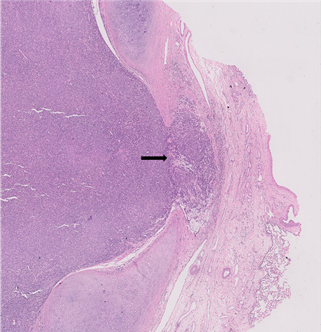
Figure 6. Tumour
infiltrates bronchial wall microscopically (arrow).
Discussion
The term "glomus" originates from Latin,
meaning "ball" or spherical mass. Oide et al. analysed
reported cases of bronchopulmonary Glomus tumours, identifying 36 cases arising
from either the bronchus or lung; this series showed that the tumour occurred
predominantly in adult males (3). A PubMed search for "Glomus tumour of
uncertain malignant potential" and "bronchopulmonary Glomus
tumours" yielded two additional case reports (4), (5) and a series of 11
cases (6) from 2010 to 2019.
Radiologically, GT present as variable-sized lesions
on chest radiographs. Cunningham JD et al (7) reviewed imaging
findings in 21 cases, noting that both benign and malignant tumours appear as well-circumscribed
solid masses without internal calcification or cavitation. On CT, tumours
exhibit peripheral vascularity with heterogeneous peripheral contrast
enhancement and a lack of central enhancement in both benign and malignant
lesions. PET-CT is of limited use in differentiating benign from malignant
primary lesions, as both may show absent or low-to-moderate FDG uptake.
One of the primary challenges in evaluating Glomus
tumours is assessing their malignant potential. The recent WHO classification
(2) categorizes Glomus tumours into benign, GTUMP, and malignant based on
atypical features: Benign tumours show classic histology and lack atypical
features; malignant tumours show marked nuclear atypia or atypical mitosis;
GTUMP do not fulfil the criteria for malignancy but have at least 1 atypical
feature other than nuclear pleomorphism. This classification of GTUMP has evolved
from earlier criteria by Folpe et al (9)., with emphasis on nuclear
pleomorphism and other atypical features which include: infiltrative growth
pattern, high cellularity, necrosis, spindled morphology, large size (>2cm),
and deep location. However, cases lacking nuclear pleomorphism despite atypical
features pose challenges in exact categorization using WHO criteria. This case,
with focal moderate atypia but large size, deep location, and bronchial wall
infiltration, was categorized as GTUMP. Glomus tumours are consistently
positive for SMA; additional positive stains include vimentin, h-Caldesmon and
stromal collagen-IV. CD34 positivity has been reported in up to 32% of cases
(8). In these cases, a negative STAT6 can exclude a solitary fibrous
tumour.
Complete surgical excision with negative margins has
been recommended (9,10) for all cases. Chemotherapy has been employed in rare
cases of metastatic disease, following protocols similar to sarcomas (11).
Molecular studies of glomus tumours arising in various sites have shown the
occurrence of NOTCH gene fusions in more than half the cases (predominantly in
benign GT), less commonly, BRAF V600E and very rarely KRAS G12A mutations
(12,13). Most benign NOTCH-fusion positive GT occurred in extremities while the
malignant NOTCH positive tumours occurred in viscera (gastrointestinal tract
and lung). The identification of NOTCH gene rearrangement by FISH can help in
diagnosing a malignant glomus tumour in diagnostically challenging cases (12).
However, to the best of our knowledge, molecular markers that predict malignant
behaviour in GT have not been established so far; such a marker could aid in
cases of partially resected tumours and tumours with uncertain malignant
potential for deciding further treatment and follow-up. Next generation
sequencing of these tumours may facilitate identification of further molecular
alterations and thereby, potential targets for therapy.
Conclusion
Primary pulmonary Glomus tumors are rare lesions in
the bronchi or lungs, often mistaken for more common entities at these sites
and challenging to differentiate radiologically. Glomus tumours can exhibit
atypical or malignant features, necessitating comprehensive evaluation of all
specimens. Ambiguities persist in exact categorization of Glomus tumours with
uncertain malignant potential. While complete surgical excision remains the
preferred treatment, alternative options such as chemotherapy and targeted therapy
need to be explored. The identification of molecular markers to predict
malignancy and potential targets for treatment warrant further studies.
Etical approved
Written informed consent for publication was obtained
from the patient.
Author
contribution
Conceptualization: ShS, ShV, AK. Data curation:
ShS, ShV, AK, SK. Formal analysis: ShS, ShV, AK.
Investigation: SV, DV, AK. Methodology: ShV, AK. Administration:
ShV, AK. Resources: ShV. Software: ShV. Supervision: ShV,
AK. Validation: ShV, AK. Visualization: ShV, AK.
Writing—original draft: ShS, ShV. Writing— review & editing: ShV,
AK. Approval of final manuscript: all authors..
Conflict
of interest
There
is no Conflicts of interest/competing interests.
Funding
There
is no funding.
References
1. Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab
Med. 2008 Sep;132(9):1448-52.
2. Specht K, Antonescu CR. Glomus tumour. In:
WHO classification of tumours (5th edition) of soft tissue and bone.
Lyon: International Agency for Research on Cancer (IARC). 2020:179–181.
3. Oide T, Yasufuku K, Shibuya K, Yoshino I, Nakatani
Y, Hiroshima K. Primary pulmonary glomus tumor of uncertain malignant
potential: A case report with literature review focusing on current concepts of
malignancy grade estimation. Respir Med Case Rep. 2016;19:143-9.
4. Jin Y, Al Sawalhi S, Zhao D et al. Behavior of
primary tracheal glomus tumor, uncertain malignant potential subtype. Gen
Thorac Cardiovasc Surg. 2019 Nov;67(11):991-5.
5. Dai SH, Tseng HY, Wu PS. A glomus tumor of the lung
of uncertain malignant potential: A case report. Int J Clin Exp Pathol. 2018
Dec;11(12):6039-41. Erratum in: Int J Clin Exp Pathol. 2019 Mar;12(3):1117.
6. Zhao SN, Jin Y, Xie HK, Wu CY, Li Y, Zhang LP.
Clinicopathological characterization of primary pulmonary and tracheal glomus
tumors. Zhonghua Bing Li Xue Za Zhi. 2020 Dec 8;49(12):1282-7.
7. Cunningham JD, Plodkowski AJ, Giri DD, Hwang S.
Case report of malignant pulmonary parenchymal glomus tumor: Imaging features
and review of the literature. Clin Imaging. 2016 Jan;40(1):144-7.
8. Mravic M, LaChaud G, Nguyen A, Scott MA, Dry SM,
James AW. Clinical and histopathological diagnosis of glomus tumor: An
institutional experience of 138 cases. Int J Surg Pathol. 2015;23(3):181-8.
9. Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW.
Atypical and malignant glomus tumors: Analysis of 52 cases, with a proposal for
the reclassification of glomus tumors. Am J Surg Pathol. 2001 Jan;25(1):1-2.
10. Wood C, et al. Malignant glomus tumors: Clinical
features and outcomes. Clin Sarcoma Res. 2020;10:20.
11. Wolter NE, Adil E, Irace AL, et al. Malignant
glomus tumors of the head and neck in children and adults: Evaluation and
management. Laryngoscope. 2017 Dec;127(12):2873-82.
12. Agaram NP, Zhang L, Jungbluth AA, Dickson BC,
Antonescu CR. A molecular reappraisal of glomus tumors and related pericytic
neoplasms with emphasis on NOTCH-gene fusions. Am J Surg Pathol. 2020
Nov;44(11):1556-62.
13. Chakrapani A, Warrick A, Nelson D, et al. BRAF and
KRAS mutations in sporadic glomus tumors. Am J Dermatopathol. 2012;34: 533–535.