Investigation of exosomes in aged human fibroblasts
cultured in serum-free medium
Nadia Ghobeishavi 1, Amrolah Mosfatazade 2,
Bagher Seyedalipour 3 *
1 Department of Cellular and Molecular
Sciences, University of Mazandaran, Babolsar, Iran
2
Department of Immunology, School of Medicine, Babol University of Medical
Sciences, Babol, Iran
3 Department of Molecular and Cell
Biology, Faculty of Basic Science, University of Mazandaran, Babolsar, Iran
Corresponding
Authors: Bagher Seyedalipour
* Email: b.seyedalipour@umz.ac.ir
Abstract
Introduction: Exosomes are small vesicles (30 to 100 nm)
crucial for intercellular communication and influence various biological and
pathological processes. This study examined exosome secretion in human skin
fibroblasts in vitro.
Materials and methods: Supernatants from young fibroblasts (passage 3) and aged
fibroblasts (passage 12) cultured in DMEM medium, with or without 10% fetal
bovine serum (FBS), were collected for analysis. After confirmation of exosome
presence by scanning electron microscopy, the number of exosomes was measured
using flow cytometry with magnetic beads coated with a specific antibody
(anti-CD81). Additionally, the protein profile of these exosomes was examined
using SDS-PAGE.
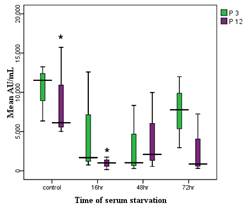
Results: Electron microscopy revealed exosomes with diameters from 33 to 93 nm
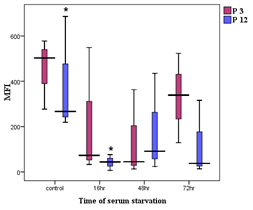
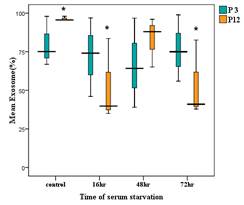
in fibroblast culture supernatants. Aged fibroblasts showed a significantly
reduced abundance of exosomes in serum-starved conditions at 16 and 72 hours
compared to controls (P<0.05). However, there was no significant difference
in exosome abundance between young and aged fibroblasts in serum conditions.
Young fibroblasts exhibited no significant differences in exosome levels across
serum-starved and control groups at various time points. The concentration and
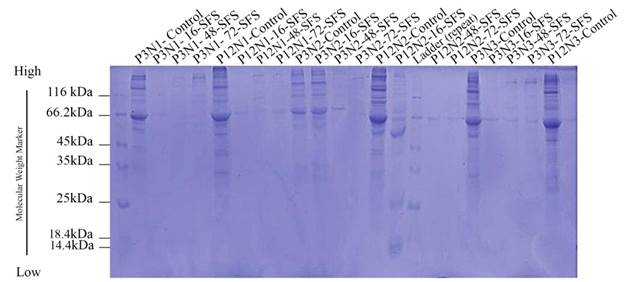
mean fluorescent intensity (MFI) supported these findings. Electrophoresis
showed exosome proteins ranging from 14 to 116 kDa,
with no significant differences between age groups. Protein band density in
serum-starved exosomes was lower than in controls, with only one exception
noted.
Conclusions: In aged fibroblasts, there were no significant changes in exosome
quantity or protein patterns, but under serum-starved conditions, notable
differences arose. The overall number of exosomes decreases under serum
deprivation, though not significantly, while protein band density significantly
decreases. Aged cells continue to secrete exosomes in serum deprivation, but
their protein content diminishes. This finding improves our understanding of
wound healing, cancer, and cell therapy.
Keywords: Exosomes, Cellular Senescence, Fibroblasts, Serum Deprivation
Introduction
Cellular
senescence refers to a permanent halt in the cell cycle, which arises from the
limited capacity for cell replication, particularly in normal human fibroblasts
(1). This
phenomenon is believed to contribute to the aging process of fibroblasts.
Various factors influence the aging process, including genetic background,
genomic instability, free radical production, changes in telomerase enzyme
activity, dietary restrictions, waste product accumulation, DNA methylation,
stress, mitochondrial mutations, DNA damage and repair, DNA-protein
interactions, histone acetylases, and histone deacetylases (2, 3). Senescent
cells can be differentiated from other non-proliferative cells through specific
markers and morphological changes. These changes include the absence of the
proliferative activity marker SAβGAL, the expression of pro-inflammatory
factors like IL-6 and IL-8, the presence of chemokines, cell cycle inhibitors,
tumor suppressors, and signs of DNA damage. The theory of cellular senescence,
also known as the Hayflick limit, was first proposed by Leonard Hayflick and
Paul Moorhead (4). It is
important to note that aging manifests at multiple levels: organ, tissue, cell,
and molecular. Senescent fibroblasts build up in older organisms, especially in
certain tissues. The highest concentrations of these fibroblasts have been
observed in the skin, liver, lungs, and spleen. Fibroblasts are among the most
abundant cell types in the body, especially within connective tissues, and play
a crucial role in wound healing and the aging process (5). As organisms
age, these fibroblasts build up in the lower layer of the skin and secrete
substances typically released only during wound healing. These substances
include collagenase and elastase, which are matrix metalloproteinases that
degrade elastin and collagen in the skin (6). In addition
to the above functions, senescent fibroblasts secrete enzymes that can degrade
the basement membrane, which is vital for the proper organization and function
of epithelial cells. Other substances secreted by fibroblasts include TGFβ,
insulin-like growth factor-binding protein 1 (IGF1), PAI1, inflammatory
cytokines, and decreased levels of lamin B1, VEGF,
and matrix metalloproteinases. These changes allow senescent fibroblasts to
communicate with each other and their surrounding environment (7). They also
release high levels of exosomes, which can alter the local microenvironment and
promote the growth and spread of nearby tumor cells (8). Some research
suggests that these changes may inhibit the spread of cancer cells, reduce cell
motility, and limit oncogenic transformations in cancer cells during early
stages. Thus, aging serves as a double-edged sword concerning cancer
development (9). Our study has
shown that when fibroblasts are subjected to serum deprivation— a form of
cellular stress— they secrete substances into the surrounding medium that
encourage fibroblast migration, as observed in scratch tests. Unpublished
findings indicate that these fibroblast secretions also enhance wound healing
in vivo (10). Given the
crucial role of exosomes in various diseases, especially cancer and autoimmune
conditions, this study aims to investigate the secretion of exosomes and
compare the proteins they contain under senescence conditions and serum
deprivation in fibroblast supernatants (11). Exosomes
secreted from cells infected with pathogens express specific antigens that
interact with the histocompatibility complex, presenting these antigens to
immune cells. Meanwhile, exosomes released from cancer cells can carry antigens
that act as both tumor-promoting and immunosuppressive agents. Besides their
regulatory role in the immune system, exosomes can stimulate tumor progression
through mechanisms such as angiogenesis.
Materials and methods
Sampling
Nine
foreskin samples were collected from newborns with an average age of 2 months
during circumcision at Babol Clinic in Babol City. The procedures were carried
out under completely sterile conditions. The samples were placed in a culture
medium consisting of 80% DMEM (PAA, Austria), 10% Penicillin/Streptomycin (PAA,
Austria), and 10% FBS (PAA, Austria), and then transferred to the culture room.
All steps for cell isolation were conducted under a laminar flow hood using
sterile materials and equipment.
Isolation
of fibroblast cell lines from foreskin by enzymatic method
In
this method, the tissue was washed 2 to 3 times with ethanol to reduce the risk
of contamination. The samples were also washed 2 to 3 times with PBS to remove
blood cells. Next, the samples were cut into small pieces using a surgical
blade, and the slimy, bloody layer was separated. This layer was then
transferred to a Falcon tube containing the enzyme dispase
at a concentration of 5 mg/ml, maintained at 37 degrees Celsius in a water bath
for 3 hours. During this process, the epidermis layer was separated from the
dermis layer by the enzyme disease. Afterward, the dermis layer was divided
into very small pieces and transferred to a Falcon tube containing the enzyme
collagenase at a concentration of 1 mg/ml, also maintained at 37 degrees
Celsius in a water bath for 20 minutes. Collagenase breaks down collagen
proteins, allowing the cells to separate from the tissue. The supernatant
containing the separated cells was then removed and placed in a Falcon tube
with a culture medium, and collagenase was added to the remaining tissue again.
This step was repeated multiple times until all the tissue was dissolved in the
collagen solution. Subsequently, the culture medium containing the cells was
passed through a cell separation filter to create a uniform cell suspension.
The cells were then centrifuged at 1500 rpm for 7 minutes at 4°C to sediment
them. The supernatant was discarded, and the cells were homogenized in 1 ml of
culture medium. Finally, after counting the cells and checking the percentage
of viable cells, the cells were cultured in specialized cell culture flasks.
Cell
culture
Cells
isolated from the skin were placed in a 25 cm² flask, with a density of 100,000
cells per flask. They were grown in a medium made up of 89% DMEM, 10% FBS, and
1% Penicillin/Streptomycin. This setup was maintained in an incubator under
standard conditions (temperature: 37°C, 5% carbon dioxide, and 95% humidity). After
24 hours, the culture medium was discarded, the adherent cells were rinsed with
PBS, and a fresh culture medium was introduced. The culture medium was then
changed every two days until the cells covered the entire surface of the flask
continuously.
Culturing
fibroblasts in serum-free medium
Ten^5
cells (from passages 8, 4, and 12) obtained from three skin samples were placed
in a 25 cm² flask containing a culture medium composed of 89% DMEM, 10% FBS,
and 1% Streptomycin/Penicillin. The flask was incubated under standard
conditions (37°C, 5% carbon dioxide, and 95% humidity) for 48 hours until cell
attachment reached approximately 70 to 80%. After this period, the complete
culture medium was removed, and the cells were washed several times with PBS.
Subsequently, a serum-free DMEM culture medium was added to the flasks and
incubated for 16, 48, and 72 hours. Additionally, a complete culture medium
containing 10% FBS was added to one flask as a serum-containing positive
control.
Cell
culture supernatant storage
After
the specified incubation periods, the supernatant, which consisted of a DMEM
culture medium and other cellular secretions, was carefully removed. It was
then stored in 500 μl microtubes at -20°C for
subsequent experiments, including exosome extraction using the desired kit,
electron microscopy, flow cytometry, and SDS-PAGE.
Analysis
of supernatant of fibroblasts cultured in serum-free and serum-free medium
(SFS)
To
obtain a general view of the protein composition of the supernatant from
fibroblasts cultured in serum-free media (maintained at -20°C) and
serum-containing media, the electrophoretic migration pattern of proteins was
examined using sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE).
Reducing
polyacrylamide gel electrophoresis
In
this study, a discontinuous electrophoresis system was employed following the
method established by Laemmli (1970). First, a separating gel with a pH of 8.8
(see Table 2-1) was poured between two electrophoresis vials. After the gel
polymerized, a concentrating gel with a pH of 8.6 (see Table 2-2) was added on
top, and wells were formed by placing a comb in the gel. Once the gel was
completely polymerized, samples were prepared that included exosomes extracted
from both old fibroblasts (passage 12) and young fibroblasts (passage 3) at
various time intervals of serum deprivation. Additionally, exosomes from
positive control samples, which consisted of fibroblasts cultured in DMEM
containing FBS and previously concentrated using the exosome extraction kit,
were included. Each sample was mixed with a 4x sample buffer (refer to Table
2-3) in a ratio of 1:3 (sample buffer to sample), and then heated for 2 to 5
minutes. The prepared samples were loaded into the wells created in the gel.
Freshly prepared tank buffer (see Table 2-4) was used for the migration
process. Bromophenol blue served as a migration control in this technique.
Initially, a voltage of 80 V was applied while the samples were in the
concentrating gel. When the samples entered the separating gel, the voltage was
increased to 120 V and subsequently to 150 V.
Staining
polyacrylamide gel with Coomassie blue
Coomassie
blue is the most commonly used dye for staining proteins. Its advantages
include ease of use, color stability, and relatively high sensitivity, with the
ability to detect proteins in amounts ranging from 0.2 to 0.5 μg per band. In this method, the steps of fixing and
staining the proteins are conducted simultaneously.
Coomassie
Blue staining method
A
sufficient volume of dye solution was added to the gel, and then the container
was closed and placed on a shaker for 1-2 hours. Afterward, the dye solution
was drained, and the gel was thoroughly washed with plain water. Next, a dye
remover solution was added, and the container was placed on the shaker again.
This process was repeated several times until the gel background became
transparent and the protein bands were visible. In the final step, the gel was
placed in a 7% acetic acid solution, which allows for long-term storage.
Exosome
extraction
Sample
preparation
The
supernatant from fibroblasts cultured in young (3-5 passages) and old (12-15
passages) is collected and then centrifuged at 2000 g for 30 minutes. This
process removes dead cells and other debris. After the centrifugation, the
clear supernatant is carefully transferred to a new, clean microtube, making
sure not to mix it with the sediment in the original microtubes.
To
extract exosomes from the fibroblast supernatant, we utilized an exosome
extraction kit (TEI). First, we took 1 ml of the centrifuged fibroblast
supernatant prepared in the previous step and added 0.5 ml of the extraction
buffer provided in the kit, following the protocol. Next, we mixed the
supernatant and extraction buffer thoroughly by vortexing
or using a pipette to create a homogeneous solution. The microtubes containing
this mixture were then incubated at a temperature between 2-8°C for 24 hours. After
the incubation period, the samples were centrifuged at 10,000 g for 1 hour,
also at 2-8°C. Subsequently, we discarded the supernatant from the microtubes,
leaving behind a precipitate that contains the exosomes. To prepare a uniform
solution, we added 25-100 μL of PBS x1 buffer to the exosomal precipitate. The extracted exosomes can be stored
for one week at 2-8°C or for an extended period at -20°C.
Extraction
of CD81-positive exosomes
Preparation
of Assay Buffer
The
measurement buffer consists of 0.1% BSA + PBS, which has been passed through a
0.2 µm filter.
Preparation
of beads that detect exosomes with CD81 marker
Dynabeads were utilized in this study. These beads are magnetic polystyrene
beads measuring 2.7 µm in size. They are coated with a primary monoclonal
antibody against the membrane molecule CD81, which is expressed by most human
exosomes. To prepare the Exosome-Human
CD81 Flow Detection beads (Thermo Fisher Scientific,
USA), the vial was vortexed for 30 seconds to ensure a homogeneous solution.
Then, 20 µl of the bead solution was removed and added to a microtube
containing 1 µl of buffer. The buffer-bead mixture was placed on a magnet for
1-2 minutes. While the microtubes were still attached to the magnet, the
supernatant buffer was carefully removed, and 90 µl of fresh buffer was added.
After that, 10 µl of the sample containing exosomes was introduced to the
buffer-bead mixture. The resulting
mixture was then placed on a rotator and incubated at 2-8°C for 24 hours,
allowing the beads and exosomes to mix and bind to each other. After
incubation, the samples were briefly centrifuged for 1-2 seconds. Subsequently,
300 μL of Assay Buffer was added, and the mixture was
placed on the magnet for another 1-2 minutes. Afterward, the supernatant was
removed before separating the samples from the magnet. This process was
repeated after adding another 300 μL of buffer to
wash the beads attached to the exosomes for purification. In the final step, an
additional 300 μL of buffer was added to the samples,
resulting in a solution that was used for subsequent experiments, including
electron microscopy and flow cytometry.
Observation
of exosomes by scanning electron microscopy (SEM)
To
observe exosomes by electron microscopy, 10 μl of the
solution prepared in the previous step was spread on a slide and allowed to
dry. This sample was then analyzed by electron microscopy (SEM).
Flow
cytometry
In
this step, 100 µL of the exosome sample bound to the beads is added to a
microtube, followed by the addition of 20 µL of monoclonal antibody CD81 (Mouse
anti-human CD81-PE, BD Bioscience, USA). The samples are subsequently placed on
a shaker set at 1000 rpm and incubated at room temperature for 45 minutes.
During this incubation, it is important to keep the samples away from light and
in a dark environment. After 45 minutes, 300 µL of buffer is added to each
sample, which is then placed on a magnet for 1-2 minutes. The samples are
washed by first removing the supernatant before separating them from the
magnet. Afterward, 300 µL of buffer is added again, and this process is
repeated for a thorough wash. In the final step, an additional 300 µL of buffer
is added. The volume of buffer in the final step may vary depending on the
specific flow cytometry device used, and it can be adjusted up to 1 mL. Once prepared, the samples are analyzed using a BD FACSCalibur device.
Data
analysis
The
obtained data were analyzed and interpreted using SPSS 16 statistical software,
Microsoft Excel, and Flowjo software, and P<0.05
was considered a significant difference.
Results
Results
from exosome extraction
To
investigate the secretion of exosomes by fibroblasts isolated from human skin
under conditions of cellular stress, we collected equal volumes of supernatants
from fibroblasts cultured in both serum-free and serum-containing media. The
exosomes from these supernatants were then concentrated and extracted using the
Total Exosome Isolation Kit (Thermo Fisher
Scientific, USA). The figure below (Figure 1) shows that exosomes are
present in the fibroblast supernatants, even under serum-deprived conditions.

Figure
1. Pellet
obtained by centrifugation of equal volumes of control (right) and
serum-starved (left) fibroblast supernatants.
Results
from scanning electron microscopy (SEM)
To
investigate the morphology of exosomes and to prepare a positive control sample
for flow cytometry, we prepared exosome sediment from the supernatant of
fibroblasts cultured in a serum-containing medium. This sample was sent to the
Rezaei Electron Microscopy Laboratory in Tehran for scanning electron
microscopy (SEM) imaging. Figure 2 illustrates the presence of exosomes
in the fibroblast supernatant, with sizes ranging from 33 to 92 nm.

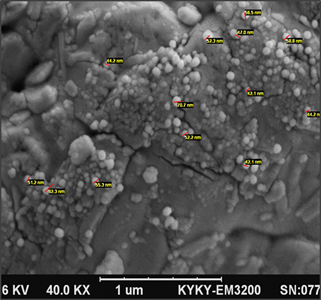
Figure
2.
Results from scanning electron microscopy of exosomes.
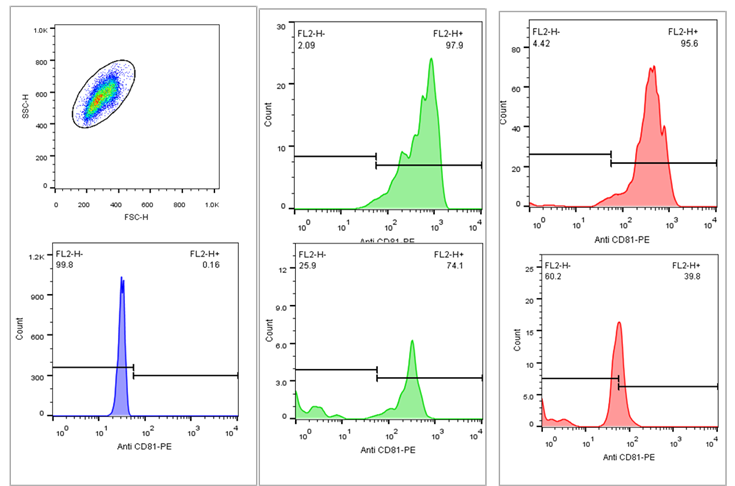
Flow
cytometry results
For
semiquantitative measurement of exosomes, the supernatant from fibroblasts was
purified using magnetic beads that contain antibodies specific to the CD81
marker. This was followed by labeling with a secondary antibody conjugated to a
PE fluorescent marker. The analysis was conducted using a Becton-Dickinson
instrument, focusing on the FL2 channel. The figure below illustrates the
presence of CD81-positive exosomes in the supernatant of fibroblasts under both
serum-containing and serum-free culture conditions. As shown in the image, the
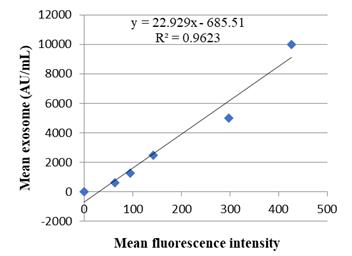
quantity of exosome production varies and is notably reduced in serum-free
culture conditions (Figure 3).