The
co-administration of quercetin and gallic acid nanocapsules
exhibits a protective effect against aluminium
chloride in the brain of animal model
Reza
Taghizadeh-Tabarsi 1, Alimohammad
Madih 2, Mohammad Gilanifar 2, Fatemeh Zahra Gharib 3,
Ali Fakhrtavoli 2, Amirhossein
Esmaeilzadeh 2, Ali Taravati 4*
1 Faculty of Life Science and
Biotechnology, Shahid Beheshti University, Tehran, 16589-53571, Iran
2 Department of Veterinary Medicine,
Islamic Azad University, Babol Branch, Tehran, Iran
3 Department of Clinical Sciences, Bab.C., Islamic Azad University, Babol, Iran
4
Department of Molecular and Cell Biology, Faculty of Basic Sciences,
University of Mazandaran, Babolsar 47416-95447, Iran
* Corresponding Author:
Ali Taravati
* Email: a.taravati@umz.ac.ir
Abstract
Introduction: Aluminum (Al) is
associated with the development of various neurological disorders, including
Alzheimer's disease (AD), highlighting the need for materials with protective
effects. This study investigated the protective effect of quercetin and gallic
acid nanocapsules on brain damage caused by aluminum
chloride.
Materials and methods: Adult rats were chronically treated with aluminum chloride to generate
a disease model. Gallic acid and quercetin were administered orally, both in
free forms and as nanocapsules, to evaluate their
protective effects. To assess oxidative stress, the levels of lipid
peroxidation, total antioxidants, reduced glutathione, glutathione peroxidase,
superoxide dismutase, catalase, and myeloperoxidase activity were measured.
Brain tissue was also examined for structural abnormalities using hematoxylin
and eosin staining.
Results: Aluminum chloride treatment significantly increased oxidative stress
and brain damage. However, treatment with a combination of gallic acid and
quercetin, both in free (20 mg/kg and 50 mg/kg, respectively) and nanocapsule forms, effectively reduced these effects.
Histological evaluation showed that co-treatment with quercetin and gallic acid
nanocapsules significantly reduced aluminum-induced
toxicity and preserved normal brain structure. The nanocapsule
forms were more effective at lower doses (10 mg/kg) compared to the free forms.
Conclusion: These findings suggest that quercetin and gallic acid nanocapsules can reduce the required therapeutic dose and
limit the adverse effects of the free drugs. Nanocapsule
formulations may enhance brain delivery and act as neuroprotective agents
against aluminum-induced damage and the progression of Alzheimer’s disease. The
encapsulated form of quercetin and gallic acid appears to be a promising
protective agent in preclinical evaluations.
Keywords: Alzheimer's disease, Aluminium chloride,
Quercetin, Gallic acid, Nanocapsules
Introduction
Alzheimer's disease is one of the neurodegenerative diseases that
affected more than 55 million people in the world in 2020. This number will
double approximately every 20 years, reaching 78 million in 2030 and 139
million in 2050 (1, 2). Alzheimer's often occurs in people over 65 years of age, but
about 10% of patients develop early-onset Alzheimer's and develop this
condition in their 30s to 60s (2). Also, women suffer from Alzheimer's more than men (3). So far, there is no known way to stop or prevent the progress of
this disease, but some treatments help to improve the symptoms of the disease.
Aluminum, as a metal that can cause Alzheimer's, puts humans at risk with
different sources. The equipment is present in water, food, environment,
medicinal compounds, etc., and it is placed in the soil due to acid rain.
Disorders such as dementia (brain damage) in Alzheimer's disease as well as
prevention of motor actions in Parkinson's disease in humans and animals are
related to increasing consumption. Aluminum can cause inflammatory damage to brain tissue
by causing oxidative stress (4, 5). Therefore, preventing oxidative stress and generating a
protective effect using antioxidants has an important place. Gallic acid has effective antioxidant
properties as a trihydroxybenzoic acid and a phenolic
acid with a molecular weight of 170.12 g/mol. This compound is found in sumac, hazelnut,
tea leaves, oak bark, and other plants. Gallic acid acts as an antioxidant and
helps protect cells from oxidative damage. It has been observed that gallic
acid has anti-cancer properties. It is also used in the treatment of internal
bleeding. It is also used as medicine in the treatment of albuminemia and
diabetes. Gallic acid plays a neuroprotective role in animal models that have
the problem of nerve damage through pathways that include antioxidant and
anti-inflammatory activity. Gallic acid has a spectacular protective effect on
neurotoxicity and neurotoxicity that is caused after brain damage (6). Quercetin, as a flavonol from the
group of flavonoid polyphenols, has antioxidant properties that can be found in
many fruits, vegetables, leaves, seeds, and grains such as red onion and kale (1). This substance is one of the most abundant flavonoids in the
diet with an average daily consumption of 50 mg (7). Quercetin, like other flavonoids, can cross the blood-brain
barrier (BBB), which makes it a potential agent in preventing neurodegenerative
disorders (8). Flavonoids widely have anti-inflammatory and antioxidant
activity, both of which are effective in preventing Alzheimer's pathogenesis.
Quercetin can treat many problems, including neurological disorders, and delay
the process of nerve damage. This substance also has antioxidant and protective
effects in preventing endothelial apoptosis caused by oxidants (9). The effectiveness of the drug in the central nervous system
depends on the ability of the drug to cross the blood-brain barrier and reach
therapeutic concentrations in the brain after administration. Therefore,
failure in the treatment of central nervous system disorders is often not due
to the lack of potential effect of the drug, but due to problems in the method
of drug delivery (10). To overcome the problems and obstacles of drug delivery,
nanoparticles and nanocarriers are being developed that can deliver drugs in a
targeted manner and increase the effectiveness of drugs in a wide range of
diseases from cancer to Alzheimer's (11, 12). Chitosan, as an alkaline polysaccharide that is biocompatible,
can be effective in drug delivery because it can prevent enzymatic degradation.
Since the nanocapsulation of the drug with chitosan
can increase the passage through the blood-brain barrier, this carrier provides
good conditions for drug delivery to brain cells (13, 14). Therefore, our hypothesis was that co administration of gallic
acid and quercetin, particularly in a nano encapsulated form, would
synergistically reduce oxidative stress and neuroinflammation in a rat model of
aluminum chloride induced Alzheimer’s model more effectively than either
compound alone and that this combination can be effective even at lower doses
due to improved bioavailability from nanocapsulation,
based on this hypothesis, this study
aimed to investigate the biochemical and histological effects of using
chitosan-alginate nanocapsules with quercetin and
gallic acid to treat or prevent aluminum chloride-induced brain damage and
lesions. Through this research, we developed a combination therapy using
quercetin and gallic acid nanocapsules by gavage to
protect and prevent aluminum-induced brain damage in rat models.
Materials and
methods
Nanocapsulation of gallic acid and quercetin
To generate of gallic acid and quercetin
nanocapsules, gallic acid solution with a
concentration of 50 mg/ml in ethanol and a solution of quercetin with a
concentration of 6 mg/ml in DMSO and separately by chitosan with a
concentration of 0.8 mg/ml and pH 5.4 were mixed (chitosan-drug) gently on
stirrer (500 rpm). Separately, calcium chloride with a concentration of 3.35
mg/ml was slowly added to the 3 mg/ml alginate solution with a pH of 5.1, and
then the chitosan-drug solution was slowly added to it (on stirrer 500 rpm). Then, the final drug nanocapsules
solution was centrifuged at 13,000 rpm and the precipitate was dried with a
freeze dryer. To evaluate the encapsulation efficiency, the presence of
Quercetin and Gallic acid was checked by evaluating the supernatant absorption
in 375nm for Quercetin and 270nm for Gallic acid.
Characteristics
of quercetin and gallic acid nanocapsules
To determine the dimensions of the
quercetin and gallic acid nanocapsules, the
freeze-dried nanocapsules were dissolved in water.
The size of the nanoparticles was then measured using a DLS device at a
temperature of 25°C and a scattering angle of 90 degrees.
Hemolysis
assay of nanocapsules
To evaluate the effect of gallic acid
and quercetin nanocapsules on the lysis of red blood
cells (RBC), human blood was centrifuged at 500 rpm and after washing with PBS,
the RBCs were separated and incubated with gallic acid (50 mg/ml) and quercetin
(20 mg/ml) for 3 h in 37C. After incubation, they were centrifuged at 1000 rpm
and the absorbance of the supernatant was measured at 540 nm. Triton-X100 was
used as a positive control and PBS buffer was used as a negative control.
% Hemolysis = [ (Asample
– Ablank) / (Atriton
– Ablank) ] × 100
Animals
and study design
To investigate the protective effects of gallic acid and quercetin nanocapsules in rats, a total of 36 maturated male rats weighing between 250-300
grams were divided into 6 groups, each including 6 rats. The rats were
subjected to a 12-hour light and dark cycle and were provided with unrestricted
access to food and water (ethical approve code is IR.IAU.BABOL.REC.1400.028).
Condition of each group shown below (Table 1).
Table1. Treatment groups.
|
The first group, as a control, was fed normal saline orally for 35
days.
|
|
The second group, as a positive control, rats were induced Alzheimer
with aluminum chloride at a dose of 75 mg/kg by intraperitoneal injection for
35 days.
|
|
The third group was given gallic acid at a dose of 50 mg/kg and
quercetin at a dose of 20 mg/kg was consumed as a daily cocktail for 35 days
(by gavage).
|
|
Fourth group were fed gallic acid and quercetin nanocapsules
with a dose of 10 mg/kg for 35 days (by gavage).
|
|
Fifth group received quercetin and gallic acid cocktail (in the form
of gavage) with doses of 20 and 50 mg/kg, respectively, along with aluminum
chloride (IP) with a dose of 75 mg/kg.
|
|
The sixth group of rats received the combined cocktail of quercetin
and gallic acid nanocapsules (in gavage) at a dose
of 10 mg/kg along with aluminum chloride (IP) at a dose of 75 mg/kg for 35
days.
|
The first group, as a control, was fed
normal saline orally for 35 days. In the second group, as a positive control,
rats were induced Alzheimer's with aluminum chloride at a dose of 75 mg/kg by
intraperitoneal injection for 35 days. The third group was given gallic acid at
a dose of 50 mg/kg and quercetin at a dose of 20 mg/kg was consumed as a daily
cocktail for 35 days (by gavage). The fourth group was fed gallic acid and
quercetin nanocapsules with a dose of 10 mg/kg for 35
days (by gavage). The fifth group received a quercetin and gallic acid cocktail
(in the form of gavage) with doses of 20 and 50 mg/kg, respectively, along with
aluminum chloride (IP) with a dose of 75 mg/kg. The sixth group of rats
received the combined cocktail of quercetin and gallic acid nanocapsules
by gavage at a dose of 10 mg/kg along with aluminum chloride (IP) at a dose of
75 mg/kg for 35 days.
Histopathological
study
To evaluate the protective effect of quercetin
and gallic acid nanocapsules by gavage on the cortex
and hippocampal tissues, after the period of medication, the animals were
deeply anesthetized with a high dose of ketamine (150 mg/kg) and xylazine (15
mg/kg), then they were prepared for tissue sampling. Then, the animal's brain were removed and after fixing the sample, tissue passage
steps including dehydration, alcohol extraction, paraffin immersion, and
molding were performed, and using a microtome, slices with a diameter of 5 to 7
µM were removed, and stained by Hematoxylin and Eosin. With an optical
microscope, the structure and cellular morphology of the target tissue was
examined.
Evaluation
of oxidant and antioxidant parameters
Measurement of tissue stress markers
such as MDA, TAC, SOD, catalase, glutathione peroxidase, reduced glutathione,
and myeloperoxidase were measured and analyzed after the preparation of tissue
homogenate, according to standard instructions. All the tissues were kept in a
freezer at -80 degrees Celsius until the time of work. The frozen tissues were
carefully weighed and homogenized in phosphate-buffered saline. After that, the
samples were centrifuged at a temperature of 4 degrees Celsius for 15 minutes.
The supernatant solution was used to measure the desired biochemical marker
with commercially available kits (Navand Salamat,
Iran).
Statistical
analysis
All evaluations were done in three
independent replications, and results analyzed by Graph pad prism 8 and SPSS
with one way ANOVA method. The significance threshold was consider
0.05 for p-value.
Results
Size
measurement of nanocapsules
The size of gallic acid and quercetin nanocapsules was evaluated by DLS device. As shown in
Figure 1A, the sizes of quercetin and gallic acid nanocapsules
were 135 and 161 nm, respectively.
Hemolysis
assay of nanocapsules
The effect of generated nanocapsules on RBC, was investigated with a hemolysis
test. As shown in Figure 1B, gallic acid and quercetin nanocapsules
do not induce lysis of RBCs.
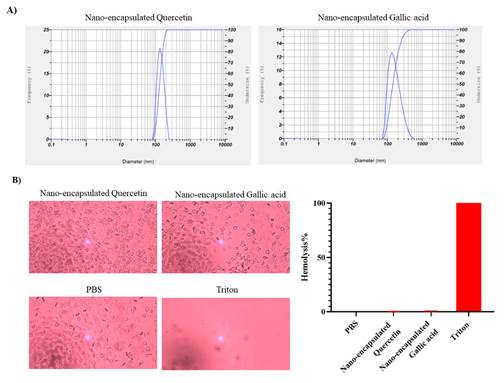
Figure 1.
Characterizing the size and effect of hemolysis of drug nanocapsules. A) The size of nanocapsules
was measured by DLS device, quercetin and gallic acid nanocapsules
were 135 and 161 nm, respectively. B) Quercetin and gallic acid nanocapsules do not lyse RBCs, PBS was used as a positive
control and Triton X100 was used as a positive control (Magnification 20X).
Histopathological
assessment
To investigate the protective effect of
gallic acid and quercetin and their nanocapsules
forms against the damage caused by aluminum chloride, the histopathology of the
cortex and hippocampus tissue of the brain under normal conditions and after
drug administration has been done with hematoxylin-eosin staining. As shown in
Figure 2, the cortex tissue in the negative control group has normal
conditions. In the group that received the cocktail of both
gallic acid and quercetin nanocapsules by gavage, the conditions
were normal, but the group that received the cocktail of gallic acid and
quercetin by gavage had symptoms of hematuria. The group receiving aluminum
chloride as a positive control group shows signs of necrosis and gliosis. The
group that received the cocktail of gallic acid and quercetin nanocapsules, and aluminum chloride as a damage inducer,
also has necrosis and gliosis, but hyperemia has not seen. The group that
received both the gallic acid and quercetin cocktail by gavage, and aluminum
chloride, showed necrosis, hyperemia, and gliosis, all three together. As shown
in Figure 3, hippocampal tissue in the negative control group shows normal
conditions. Also, normal tissue conditions without any necrosis and hyperemia
can be seen in the group receiving the cocktail of both gallic acid and quercetin nanocapsules by gavage. The group receiving the gallic acid
and quercetin cocktail generally has hyperemia and necrosis. The positive
control group shows necrosis and hyperemia by receiving aluminum chloride. The
group receiving quercetin and gallic acid nanocapsules
cocktail and AlCl3 as a damage inducer shows reduced necrosis and hyperemia
compared with the positive control group. The group receiving the gallic acid
and quercetin cocktail by gavage, and aluminum chloride as a damage inducer,
completely shows necrosis and hyperemia.
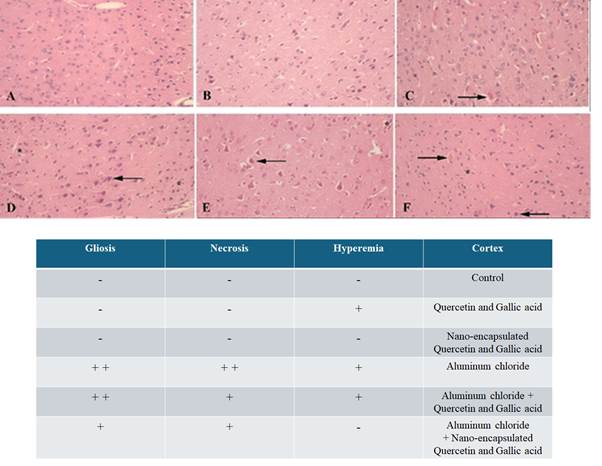
Figure 2. Investigating the protective effect of
the drug in free and nanocapsule forms against
aluminum chloride damage to the cortex. A) Normal conditions of the cortex
tissue in the negative control group. B) Normal tissue conditions in the group
receiving quercetin and gallic acid nanocapsules by
gavage, without necrosis and hyperemia. C) The group receiving gallic acid and
quercetin cocktail is hyperemic. E) The group receiving the cocktail of both
quercetin and gallic acid nanocapsules + aluminum
chloride, flash shows necrosis and the star shows gliosis, F) The group
receiving the cocktail of gallic acid, quercetin and aluminum chloride, The
left flash shows necrosis, the right flash shows hyperemia and the star shows
gliosis.
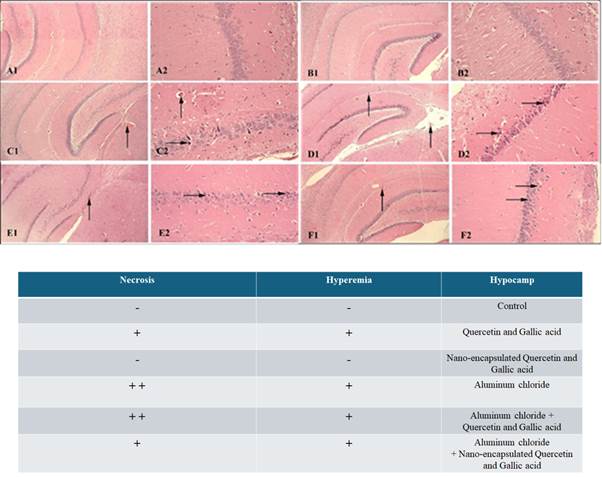
Figure 3. Investigating the protective effect of
the drug in free and nanocapsule forms against
aluminum chloride damage to the hippocampus. A) Normal tissue conditions in the
negative control group. B) normal tissue conditions in the group receiving the
cocktail of both quercetin and gallic acid nanocapsules
by gavage, without necrosis and hyperemia, C) the group receiving the cocktail
of gallic acid and quercetin by gavage in the free form, upper side flash shows
blood and the right flash shows necrosis. D) The positive control group
receiving aluminum chloride, the right flash shows necrosis and the upper side
flash shows hyperemia, and it shows more normal tissue conditions than image F,
F) group receiving quercetin, gallic acid and aluminium
chloride, the right flash indicates necrosis and upper flash indicates
hyperemia.
Oxidant
and antioxidant
In order to investigate the protective
effect of gallic acid and quercetin in free and nanocapsule
form against the damage caused by aluminum chloride, the level of oxidative
stress markers was investigated. As shown in Figure 4A, the amount of
malondialdehyde (MDA)
in the control group, free form of drug, nanocapsules
drug, aluminum chloride, aluminium chloride + free
form of drug, and aluminium chloride + nanocapsules drug were, 2.70±0.32,
2.36±0.24, 2.51±0.30, 8.48±0.56,
2.86±0.32, 2.71 ±0.28, respectively. MDA, in the group that received aluminum chloride
increased significantly compared to the negative control group
(p-value<0.001). Gallic acid and quercetin in both nanocapsules
and free forms significantly reduced MDA levels in rats treated with aluminum
chloride (p-value<0.001). As shown in Figure 4B, the amount of superoxide
dismutase (SOD)
in the group of, control, free form of drug, nanocapsules
drug, aluminum chloride, aluminium chloride + free
form of drug and aluminium chloride + nanocapsule drug were, 2.27±0.39,
2.13±0.22, 2.12±0.19, 0.87±0.10,
1.52±0.26, 2.26 ±0.36, respectively. SOD in the group of rats that received aluminum
chloride significantly decreased compared to the negative control group (p-value<0.001). Also, rats treated with aluminum
chloride as a damage inducer, when they are treated with nanocapsules
form of quercetin and gallic acid by gavage, have a significant increase
compared to treatment with quercetin and gallic acid in free form and are
closer to the negative control group (p-value= 0.001), which shows that the nanocapsules form is more effective than free quercetin and
gallic acid. As shown in Figure 4C, the amount of TAC in the group of, control,
free form of drug, nanocapsules drug, aluminum
chloride, aluminium chloride + free form of drug and aluminium chloride + nanocapsule
drug were, 29.45±1.56, 27.05±1.72, 27.02±2.00,
11.98±1.49, 23.90±1.91, 27.98 ±0.81 respectively. TAC in the group of rats receiving
aluminum chloride has a significant decrease compared to the negative control
group (p-value<0.001), The both gallic acid and quercetin nanocapsules can increase the TAC of rats induced with
aluminum chloride more than the free form (p-value= 0.02). As shown in Figure
4D, the amount of myeloperoxidase in the group of, control, free form of drug, nanocapsules drug, aluminum chloride, aluminium
chloride + free form of drug and aluminium chloride +
nanocapsule drug were, 0.26±0.02,
0.27±0.04, 0.25±0.02, 1.10±0.06,
0.52±0.03, 0.27 ±0.04, respectively. Myeloperoxidase in a group of rats that received
aluminum chloride has a significant increase compared to the negative control
group (p-value<0.001), and treatment with both nanocapsules
and free forms of gallic acid and quercetin by gavage causes a significant
decrease (Figure 4D). As shown in Figure 4E, the amount of catalase enzyme in
the group of, control, free form of drug, nanocapsules
drug, aluminum chloride, aluminium chloride + free
form of drug and aluminium chloride + nanocapsule drug were, 1.01±0.05,
0.94±0.08, 0.97±0.08, 0.41±0.05,
0.89±0.07, 0.89 ±0.07, respectively. Catalase enzyme in a group of rats receiving
aluminum chloride has a significant decrease compared to the negative control
group (p-value<0.001), and in all treatment groups with nanocapsules
form and the free form of gallic acid and quercetin, an increase in its amount
has been observed, but there is a significant difference between Treatment with
nanocapsules form and free form of gallic acid and
quercetin is not observed. As shown in Figure 4F-H, the amount of glutathione
peroxidase in the group of control, free form of drug, nanocapsules
drug, aluminum chloride, aluminium chloride + free
form of drug and aluminium chloride + nanocapsule drug were 14.19±0.76,
13.53±0.55, 13.21±0.49, 4.52±0.38,
12.28±0.50, 13.79 ±0.85, respectively. The amount of GSH in the group of, control, free
form of drug, nanocapsules drug, aluminum chloride, aluminium chloride + free form of drug and aluminium chloride + nanocapsule
drug is, 14.19±0.76, 13.53±0.55, 13.21±0.49,
4.52±0.38, 12.28±0.50, 13.79 ±0.85, respectively. The amount of reduced glutathione,
glutathione peroxidase, and glutathione reductase in all groups of rats
receiving aluminum chloride has a significant decrease compared to the negative
control group (p-value<0.001), on the other hand, in the groups treated with
free-form and nanocapsules of both gallic acid and
quercetin by gavage, there is a significant increase in all three markers. Note
that the nanocapsule drugs at a lower dose (10 mg/kg)
exhibit the same protective effect as the free drugs (50 mg/kg of gallic acid
and 20 mg/kg of quercetin) (Table 2).
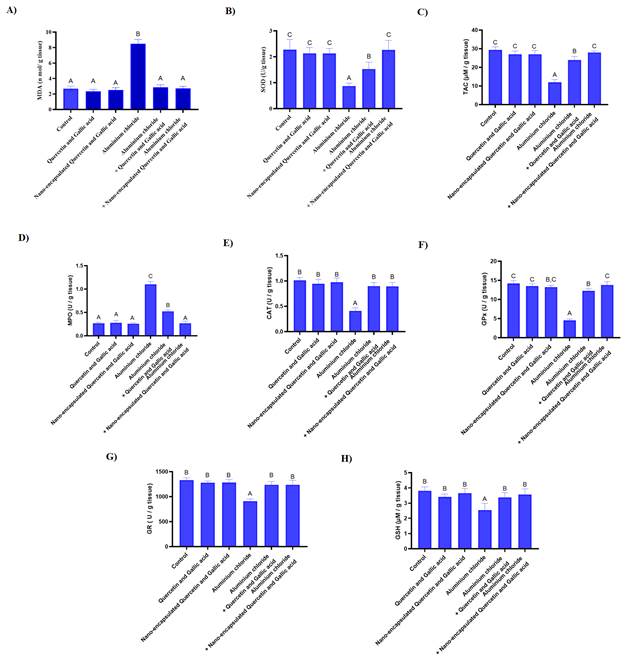
Figure 4. Investigating the protective effect of
drugs in free forms and nanocapsules on oxidative
stress. A) The amount of MDA increased following treatment with aluminium chloride, but it returned to its normal level
when treated with quercetin and gallic acid by gavage in both free and nanocapsules form. B) Aluminium
chloride reduces SOD, quercetin and gallic acid nanocapsules
significantly increasing the amount of this enzyme more than the free form. C) Aluminium chloride reduces TAC, quercetin and gallic acid nanocapsules increase the amount of SOD and return to
normal conditions. D) Aluminium chloride increases
myeloperoxidase, quercetin and gallic acid nanocapsules
decrease myeloperoxidase more significantly than the free form. E) The
treatment of both forms increases catalase and compensates for the damage of aluminium chloride. F) The nanocapsules
form more significantly compensates for the damage caused by aluminium chloride in the amount of GPx.
G) Both forms of the drug compensate for the reduction of glutathione
reductase. H) Aluminium chloride decreases GSH,
quercetin and gallic acid free and nanocapsules
compensate for the induced decrease.
Table 2. Investigating the protective effect of
drugs in free forms and nanocapsules on oxidative
stress (Data shown±SD).
|
Enzyme
|
Control
|
Quercetin and Gallic acid
|
Nano-encapsulate Quercetin and Gallic acid
|
Aluminium Chloride
|
Aluminium Chloride + Quercetin and
Gallic acid
|
Aluminium Chloride +
Nano-encapsulated Quercetin and Gallic acid
|
|
MDA
|
2.70±0.32
|
2.36±0.24
|
2.51±0.30
|
8.48±0.56
|
2.86±0.32
|
2.71±0.28
|
|
SOD
|
2.27±0.39
|
2.13±0.22
|
2.12±0.19
|
0.87±0.10
|
1.52±0.26
|
2.26±0.36
|
|
TAC
|
29.45±1.56
|
27.05±1.72
|
27.02±2.00
|
11.98±1.49
|
23.90±1.91
|
27.98±0.81
|
|
MPO
|
0.26±0.02
|
0.27±0.04
|
0.25±0.02
|
1.10±0.06
|
0.52±0.03
|
0.27±0.04
|
|
CAT
|
1.01±0.05
|
0.94±0.08
|
0.97±0.08
|
0.41±0.05
|
0.89±0.07
|
0.89±0.07
|
|
GPx
|
14.19±0.76
|
13.53±0.55
|
13.21±0.49
|
4.52±0.38
|
12.28±0.50
|
13.79±0.85
|
|
GSH
|
14.19±0.76
|
13.53±0.55
|
13.21±0.49
|
4.52±0.38
|
12.28±0.50
|
13.79±0.85
|
Discussion
Based on pre-clinical and laboratory observations, it can be stated
that aluminum chloride causes Alzheimer-like complications through the
formation of an alkylated product and the accumulation of this compound in
tissues. Additionally, it disrupts the balance between oxidants and
antioxidants in the tissue (15). However the mechanism
of action of Quercetin and Gallic acid is not completely clear, gallic acid and
quercetin both have been used as antioxidant substances and anti-inflammatory
and protective indicators in many studies. Aluminum chloride has also been used
as a substance to induce Alzheimer-like lesions in studies. In a study by Li
Yuping et al. on the effect of quercetin against Alzheimer's-inducing
beta-amyloid, quercetin was administered by gavage to mice with Alzheimer's
disease, and the behavioral and histopathological results of the brain showed
positive effects (16). Current FDA-approved treatments for Alzheimer’s disease,
such as cholinesterase inhibitors and NMDA receptor antagonists like memantine,
provide only symptomatic relief and are often associated with limited efficacy
and adverse side effects (17). In contrast, the encapsulated form of quercetin
and gallic acid, as demonstrated in our study, shows potential not only in
ameliorating oxidative stress and neuroinflammation two key contributors to
Alzheimer’s pathogenesis but also in enhancing bioavailability and sustained
release. Mowali et al. conducted a study about the
synergistic effect of quercetin and exercise against Alzheimer's
disease-induced complications in mice. For this purpose, quercetin was
administered by gavage for 60 days to mice with Alzheimer's disease. The brain
histopathology data indicated quercetin's beneficial effects in mice (18).
Takashi Mori et al. show, that gallic acid administration by gavage for 6
months daily to mice suffering from Alzheimer's disease, illustrates a good
effect on behavioral and histopathological results of the brain (19). In this
study, after the administration of aluminum chloride, a significant increase in
malondialdehyde was observed compared to the control group, which indicated an
increase in tissue damage. Furthermore, co-administration of quercetin and
gallic acid to other groups reduced the amount of this marker, demonstrating the
drug's effect both in its free form and in its nanocapsules
form, likely due to their antioxidant properties. The increase in the amount of
TAC in the treatment and control groups with drugs indicates the same issue
that the combination of both gallic acid and quercetin can benefit the body's
oxidant balance to reduce radicals or neutralize them, the group treated with nanocapsules medicine also had a significant difference
compared to the conventional treatment group (20-22). Also, in the following, we saw a decrease in
SOD enzyme in the group with aluminum chloride, which indicated an increase in
the amount of superoxides in the desired tissue,
which itself indicates an increase in free radicals and tissue oxidation and
oxidative stress, which fortunately in the treatment groups show an increase in
the amount of SOD, which can indicate both the reduction of superoxides
and the increase in the production of SOD, both of which testify to the
reduction of oxidative stress and the overcoming of the body's oxidant balance
(23). Following the administration of aluminum chloride, which destroys mainly
neutrophils, we saw a significant increase in the amount of myeloperoxidase in
the group treated with aluminum chloride compared to the control group. In the
treatment groups, we saw a significant decrease, which is caused by the
decrease in the death rate of neutrophils, which is a sign of the decrease in
tissue inflammation. The reduction of inflammation, in turn, was probably due
to the reduction of tissue stress and the return of inflammatory agents from
the tissue to the blood (5). Regarding the catalase enzyme, we saw a
significant decrease in the aluminum chloride group, which was caused by the
increase in the consumption of this enzyme to eliminate oxidative stress in the
tissue. No significant difference, regarding the catalase enzyme was observed
between treatment groups (24). In the case of glutathione, which is a strong
antioxidant and antiradical in the body and detoxifies in combination with free
radicals, a significant decrease was observed in the aluminum chloride group,
which significantly increased to the normal level in the treatment groups. This
rate was higher in the group treated with nanocapsule
by gavage form than conventional medicine, but no significant difference was
observed between these two treatments (25). Regarding the level of the two
enzymes glutathione reductase and glutathione peroxidase, it should also be
said that despite the reduction property of aluminum chloride and the significant
reduction caused by this substance in the control group, and on the other hand,
the antioxidant property and reduction of oxidative stress of these two
enzymes, there was a significant reduction. Both enzymes are in the group
treated by aluminum chloride, which are consistent with the rest of the
investigated oxidative indices. In both treatment groups, we see a significant
increase in the amount of these two enzymes compared to the control group with
aluminum chloride, which indicates the improvement of the condition and
reduction of the oxidative imbalance in the desired tissue, although, of
course, no significant difference was observed between the two treatment groups
(26). It is also important that the administration of drugs in the control
groups alone did not cause any side effects and no oxidative imbalance was
observed in any of the stress indicators, and in the dose used, there was no
effect of cell poisoning or There was no texture. In general, it should be said
that due to the oxidative properties of aluminum chloride, we are witnessing
oxidative stress in the tissue, and all the tissue stress indicators confirm
the existence of this phenomenon. On the other hand, the administration of the
drug in both the normal form and the nanocapsules has
improved the condition and returned the stress indicators to the normal state.
It is to be noted that not only nanocapsule drugs
show an increased protective effect compared to the free form of the drug, but
these effects are seen in lower doses of nanocapsule
drugs (10 mg/kg) compared to the free form of the drug (50 and 20 mg/kg). On
the other hand, it should be stated that the nanocapsules
drug was able to provide the same protective effects as the normal drug and
even better than that in a lower dose than the normal way of administering the
drug and show that the use of nanocapsules for oral
administration of the drug can improve the effectiveness and reach of the drug.
accelerate the target cells and increase the efficiency of the treatment so
that a similar result can be achieved with a lower dose of the effective
substance. While our findings highlight
the potential neuroprotective effects of encapsulated quercetin and gallic acid
against Alzheimer’s rat model, we propose to evaluate the therapeutic efficacy
of encapsulated quercetin and gallic acid in transgenic Alzheimer’s animal
models and long term evaluation of in vivo toxicity
and pharmacokinetic studies. In summary, the combination of 20 mg/kg quercetin
and 50 mg/kg gallic acid can reduce the oxidative imbalance of the tissue,
improve the oxidative stress indicators, and reduce the histopathological
lesions caused by Alzheimer's disease. Also, the nanocapsules
formed at a dose of 10 mg/kg of this drug combination can lead to better
results.
Conclusion
In this study,
the protective effects of both quercetin and gallic acid by gavage on
aluminum-induced brain damage were investigated. As the results show, the nanocapsule form of both
quercetin and gallic acid by gavage at a lower dose can protect against
aluminum-induced damage by generating a protective effect, and the nanocapsule form does not have the adverse effects that the
free form of the drug causes. Therefore, the nanocapsule
form of quercetin and gallic acid may offer a promising direction for future
preclinical studies to protect against the adverse effects of aluminum.
Author contribution
RTT, performed the experiments, collected and analyzed the data,
interpreted the findings, and wrote the initial draft of the manuscript. AM,
helped with data analysis and laboratory work and helped create figures and
tables. MG, participated in the interpretation of the results and
contributed to the biochemical and histological assessments. FZGh, controlled the data analysis procedure,
offered scientific advice and assistance with the study design, and made
significant revisions to the manuscript. AF, aided with the validation
of the results and helped prepare and characterize the nanocapsule
formulations. Technical support, statistical analysis, and manuscript revision
were all contributed by AHE. AT, supervised the entire
investigation, created the experimental setup, provided direction for
interpreting the findings, and edited the manuscript for important intellectual
content.
Funding
There is no funding.
Conflicts of interest
There are no conflicts of interest.
References
1. Tarawneh HY, et
al. Central auditory functions of Alzheimer’s disease and its preclinical
stages: a systematic review and meta-analysis. Cells. 2022;11(6):1007.
2. World Health Organization. Dementia. WHO.
2020. Available from:
https://www.who.int/en/news-room/fact-sheets/detail/dementia.
3. Vina J, Lloret A. Why women have more
Alzheimer's disease than men: gender and mitochondrial toxicity of
amyloid-β peptide. J Alzheimers Dis. 2010;20(S2):S527-S533.
4. Mahdi AA, et al. Aluminium
mediated oxidative stress: possible relationship to cognitive impairment of
Alzheimer's type. Ann Neurosci. 2010;13(1):18-24.
5. Zahedi-Amiri Z, Taravati
A, Hejazian LB. Protective effect of Rosa damascena against aluminum chloride-induced oxidative
stress. Biol Trace Elem Res. 2019;187:120-127.
6. Kahkeshani N, et
al. Pharmacological effects of gallic acid in health and diseases: A
mechanistic review. Iran J Basic Med Sci. 2019;22(3):225.
7. Formica JV, Regelson W. Review of the
biology of quercetin and related bioflavonoids. Food Chem Toxicol.
1995;33(12):1061-1080.
8. Gao H. Progress and perspectives on
targeting nanoparticles for brain drug delivery. Acta Pharm Sin B.
2016;6(4):268-286.
9. Zaplatic E, et
al. Molecular mechanisms underlying protective role of quercetin in attenuating
Alzheimer's disease. Life Sci. 2019;224:109-119.
10. Banks WA. Characteristics of compounds that
cross the blood-brain barrier. BMC Neurol. 2009;9(Suppl 1):S3.
11. Taghizadeh-Tabarsi
R, et al. Aptamer-guided graphene oxide quantum dots for targeted suicide gene
therapy in an organoid model of luminal breast cancer. Sci Rep.
2024;14(1):24104.
12. Zaki-Germi S, et al. Propolis polyphenol
nanosheet for synergistic cancer chemo-photothermal therapy. Colloids Surf A Physicochem Eng Asp. 2024;695:134262.
13. Yu S, et al. Chitosan and chitosan coating
nanoparticles for the treatment of brain disease. Int J Pharm. 2019;560:282-293.
14. Mohammadbaghban E,
et al. Oral administration of encapsulated catechin in chitosan‐alginate
nanoparticles improves cognitive function and neurodegeneration in an aluminum
chloride‐induced rat model of Alzheimer's disease. Physiol
Rep. 2024;12(13):e16095.
15. Hejaziyan LB, et
al. Effect of Rosa damascena extract on rat model
Alzheimer’s disease: a histopathological, behavioral, enzyme activities, and
oxidative stress study. Evid Based Complement Alternat Med.
2023;2023(1):4926151.
16. Li Y, et al. Activation of Nrf2 signaling by
sitagliptin and quercetin combination against β‐amyloid induced
Alzheimer's disease in rats. Drug Dev Res. 2019;80(6):837-845.
17. https://doi.org/10.1002/trc2.12179
18. Molaei A, et al. Synergistic effects of
quercetin and regular exercise on the recovery of spatial memory and reduction
of parameters of oxidative stress in animal model of Alzheimer's disease. EXCLI
J. 2020;19:596.
19. Mori T, et al. Gallic acid is a dual
α/β-secretase modulator that reverses cognitive impairment and
remediates pathology in Alzheimer mice. J Biol Chem. 2020;295(48):16251-16266.
20. Zhao R, et al. Redirected Zn
electrodeposition by an anti‐corrosion elastic constraint for highly
reversible Zn anodes. Adv Funct Mater.
2021;31(2):2001867.
21. Falode JA, et al.
Sausage tree (Kigelia africana)
flavonoid extract is neuroprotective in AlCl3-induced experimental Alzheimer’s
disease. Pathophysiology. 2017;24(4):251-259.
22. Abulfadl YS, et al.
Protective effects of thymoquinone on D-galactose and aluminum chloride induced
neurotoxicity in rats: biochemical, histological and behavioral changes. Neurol
Res. 2018;40(4):324-333.
23. Zahran ZN, et al. Electrocatalytic water
splitting with unprecedentedly low overpotentials by nickel sulfide nanowires
stuffed into carbon nitride scabbards. Energy Environ Sci.
2021;14(10):5358-5365.
24. Qusti SY, et al.
Role of combined administration of copper-nicotinic acid complex and coenzyme
Q10 against aluminium chloride–induced oxidative
stress in rat brain. Pharmacophore. 2018;9(1):19-29.
25. Liaquat L, et al. Development of AD like symptoms following co-administration of AlCl3 and
D-gal in rats: A neurochemical, biochemical and behavioural
study. Pak J Pharm Sci. 2017;30(2 Suppl):647.
26. Jayant S, et al. Pharmacological benefits of
selective modulation of cannabinoid receptor type 2 (CB2) in experimental
Alzheimer's disease. Pharmacol Biochem
Behav. 2016;140:39-50



