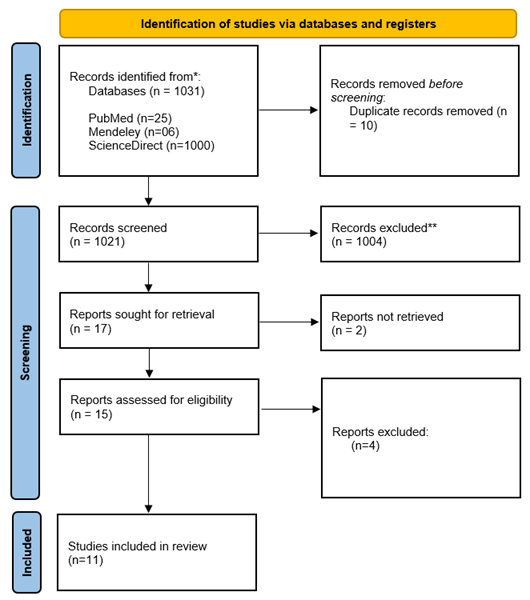
Figure 1. Prisma flow diagram illustrating the study selection process.
The relationship between antidiabetic and renal
cancer: a systematic review
Shadman Newaz 1*,
Md Hasanuzzaman 2, Ayesha Noor 3,
Abdulla Bin Hridoy 1, Talukder Nasif Shahriar 1, Noushin
Nawal 4, Anika Taseen 1, Nowshin Tasnim Khan 1,
Rahid Ahmed 1
1 Tangail Medical College, Tangail, Bangladesh
2 Rangamati Medical College, Rangamati, Bangladesh
3 Department of Pharmacy, Jahangirnagar University, Dhaka, Bangladesh
4 Institute of Applied Health Sciences, Chattogram, Bangladesh
* Corresponding
Author: Shadman Newaz
* Email: shadmannewaz11@gmail.com
Abstract
Introduction: Antidiabetic medications have been studied for potential effects beyond glycemic control, including their role in cancer
development and progression. Renal cell carcinoma (RCC) is a critical concern
in diabetic patients due to overlapping metabolic risk factors. This systematic
review evaluates the association between antidiabetic drug use and the
incidence or mortality of RCC compared to no use or alternative therapies.
Materials and methods: A systematic search was conducted across major databases to identify
observational and experimental studies examining the relationship between
antidiabetic drug exposure and RCC risk or survival. Eligible studies included
cohort, case-control, randomized controlled trials, meta-analyses, and
preclinical investigations. Data extraction focused on study design, population
characteristics, drug class exposure, renal cancer-related outcomes, and study
quality.
Results: Eleven studies met inclusion criteria. Most were observational in
nature, with one randomized trial and several meta-analyses. Evidence regarding
RCC risk and outcomes was mixed across different antidiabetic agents. Some
cohort studies indicated a potential protective association between
antidiabetic use and RCC incidence, with dose-response effects observed.
Preclinical data supported mechanistic plausibility for anticancer activity,
though human data remained inconclusive. Methodological heterogeneity—including
varied exposure definitions, follow-up durations, and confounding
adjustment—limited comparability.
Conclusion: Current evidence suggests a possible link between antidiabetic
medication use and altered RCC risk or survival, but findings remain
inconsistent and non-causal due to the predominance of observational data.
Future research should prioritize well-designed randomized controlled trials
and mechanistic studies to clarify these associations and inform personalized
therapeutic strategies.
Keywords: Metformin, SGLT2 inhibitors, Kidney cancer, Renal cell carcinoma,
Diabetes, Cancer risk, Antidiabetic medications
Introduction
Diabetes mellitus is a heterogeneous group
of disorders characterized by hyperglycemia due to
defects in insulin secretion, insulin action, or both
RCC is the most common type of kidney
cancer, accounting for over 90% of renal malignancies, and remains one of the
most lethal urological cancers worldwide
Despite increasing research, the association between antidiabetic
medication use and the risk of renal cell carcinoma (RCC) in patients with
diabetes remains inconclusive. This systematic review aims to examine whether
the use of various antidiabetic drugs, compared to no treatment or alternative
antidiabetic regimens, influences the incidence or mortality of RCC. Unlike
previous reviews that focused on specific drug classes or mechanisms, this
review adopts a broad scope, incorporating multiple study designs, diverse
antidiabetic therapies, and a range of renal outcomes. The objectives are
threefold: (1) to map the current literature on the relationship between
antidiabetic drug use and RCC, (2) to explore the long-term renal effects of
these medications given their chronic use in diabetic populations, and (3) to
identify evidence gaps that may inform future research, clinical guidelines,
and public health policies. By synthesizing the existing evidence, this review
aims to clarify the potential role of antidiabetic medications in RCC risk and
outcomes, ultimately supporting evidence-based treatment decisions.
Materials and methods
Study Design and Protocol Registration
This systematic review was conducted in
accordance with a predefined protocol registered on the Open Science Framework.
The review followed the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses (PRISMA) guidelines, ensuring transparency and thorough reporting
of the review process.
Although 11 studies were included, a
meta-analysis was not performed due to substantial clinical and methodological
heterogeneity among studies, including differences in study populations, types
and classifications of antidiabetic medications, outcome definitions, and
follow-up durations. Preliminary assessments revealed high variability in
effect measures and study designs, which would limit the interpretability of
pooled estimates. As such, a narrative synthesis was conducted in place of
quantitative meta-analysis.
Inclusion and Exclusion Criteria
The review included studies published
between January 2015 and February 2025 that examined the relationship between
anti-diabetic medications and kidney cancer outcomes. Eligible studies were of
various designs, including clinical trials, cohort studies, case-control
studies, and observational studies. Only studies published in English were
considered. Studies were included if they focused on patients diagnosed with
diabetes mellitus and explored the use of antidiabetic medications in relation
to kidney cancer outcomes. Exclusion criteria included non-English studies,
those without sufficient data for extraction, study protocols, and studies
addressing other cancer types without specific reference to kidney cancer and
antidiabetic use. Studies conducted before 2015 were excluded from the
analysis.
Search Strategy
A comprehensive and refined search was
conducted across four major electronic databases: PubMed, ScienceDirect,
Cochrane Central Register of Controlled Trials (CENTRAL), and Mendeley. The
search strategy included a combination of Medical Subject Headings (MeSH) and free-text terms designed to capture studies
related to antidiabetic medications and kidney cancer outcomes. The primary
concepts of the search were antidiabetic medications, kidney cancer, and
diabetes. Specific search terms included:
●
Antidiabetic classes: "metformin" OR "sulfonylureas"
OR "insulin" OR "glinides" OR
"thiazolidinediones" OR "DPP-4 inhibitors" OR "SGLT-2
inhibitors" OR "GLP-1 receptor agonists" OR "antidiabetic
agents."
●
Kidney cancer terms: "kidney cancer" OR "renal
cancer" OR "renal cell carcinoma" OR "kidney
carcinoma" OR "renal neoplasms."
●
Kidney cancer subtypes: "clear cell renal cell carcinoma" OR
"papillary renal cell carcinoma" OR "chromophobe renal cell
carcinoma."
Additionally, keywords such as "kidney
cancer incidence," "kidney cancer progression," "kidney
cancer recurrence," "kidney cancer mortality," and "kidney
cancer survival" were combined with terms related to antidiabetic
medications. To capture a broader range of relevant studies, terms were also
expanded to include related side effects, mechanisms, and risk assessments,
such as:
●
"diabetes treatment" OR
"antidiabetic drugs" AND "kidney cancer risk."
●
"antidiabetic side effects" AND
"kidney cancer survival."
●
"risk of kidney cancer" AND
"antidiabetic drugs."
Reference lists of key studies and reviews
were also screened to ensure no relevant studies were missed. The search
covered studies published from January 2015 to February 2025, and the database
searches were initially performed on January 26, 2025, with an update conducted
on February 26, 2025.
Screening and Data Extraction
The screening process was managed using
Rayyan software, which allowed for the removal of duplicates and facilitated
the title and abstract screening. Two independent reviewers (AH and NK)
conducted the initial screening of studies, with disagreements resolved by a
third reviewer (AT). Full-text reviews were then conducted for studies meeting
the inclusion criteria.
Data extraction was performed using a
predesigned Excel spreadsheet that captured key details, including study
design, patient population, type of antidiabetic medications used, kidney
cancer outcomes, and major findings. Data extraction was carried out by SN,
with 50% of the data verified independently by MH and NN to ensure accuracy.
Quality Appraisal
Although the primary aim of this systematic
review was to summarize and map the existing evidence rather than to critically
appraise study quality, a descriptive evaluation of study limitations and
potential biases was performed for each study. Formal quality appraisal tools,
such as the Newcastle-Ottawa Scale (for cohort and case-control studies), were
applied where appropriate, but no studies were excluded based on quality
criteria.
Data Synthesis
Due to the heterogeneity in study designs
and outcomes, a narrative synthesis was conducted. A meta-analysis
(quantitative pooling of data) was not performed due to variations in study
methods, populations, and outcome measures across the included studies. The
results were synthesized to provide a broad overview of the available evidence
on the relationship between antidiabetic medications and kidney cancer
outcomes.
Assessment of Bias
Bias assessment was carried out using
established tools and guidelines to ensure a rigorous evaluation process. The
Cochrane Risk of Bias tool was employed to systematically assess the quality
and risk of bias in the included studies. This evaluation considered various
factors, such as selection bias, performance bias, detection bias, and
reporting bias. Each study was independently reviewed by multiple researchers
to maintain consistency and objectivity in the assessment. This methodological
approach aimed to provide a comprehensive understanding of potential biases
influencing study outcomes and to enhance the reliability of the systematic
review’s findings.
Results
The study selection process for the
systematic review followed the PRISMA guidelines (Figure 1). A total of 1,031
records were identified from three databases: PubMed (25), ScienceDirect
(1,000), and Mendeley (6). After removing 10 duplicate records, 1,026 unique
records were screened. Of these, 1,009 were excluded based on title and
abstract screening.
Seventeen reports were sought for
retrieval, but two could not be accessed. The remaining 15 reports were
assessed for eligibility, with four being excluded due to irrelevance.
Ultimately, 11 studies were included in the final review. This selection process
ensured a rigorous assessment of relevant literature while minimizing bias and
maintaining study quality.

Figure 1. Prisma flow diagram illustrating the study selection process.
The studies were conducted across a range
of countries, with China contributing the highest number of studies (4),
followed by Canada with 3 and Taiwan with 2. The United Kingdom contributed to
1 study and Sweden, Denmark and Norway together
contributed to 1 study. This
distribution highlights a significant concentration of studies in Asia, Canada
and Europe, reflecting a diverse geographic spread of research.
Table 1. Country distribution of
included studies.
|
Country |
Count |
|
China |
4 |
|
Canada |
3 |
|
Taiwan |
2 |
|
United Kingdom |
1 |
|
Sweden, Denmark & Norway |
1 |
The studies varied in their methodological
designs (Table 2) which included systematic reviews with or without
meta-analysis (n=3), retrospective cohort study (n=3), cohort study (n=2),
experimental studies (n = 1), Randomized controlled trial (n=1)
& case control (n=1).
Table 2. Methodological designs of included studies.
|
Study design |
Number |
|
Systematic review and
meta-analysis |
3 |
|
Retrospective cohort study |
3 |
|
Cohort study |
2 |
|
Randomized Controlled Trial |
1 |
|
Case-control |
1 |
Key Characteristics of Included Studies
The table below (Table 3) outlines the key
characteristics of all included studies. This includes study design,
participant demographics, and specific limitations reported by each study.
Table 3. Key characteristics of studies included in the
systematic review.
|
References |
Country |
Design |
Total Participants |
Age |
Gender |
|
(11) |
Canada |
Systematic review and
meta-analysis |
7,426 patients across 9 studies |
Not specified |
Both male and female |
|
(12) |
Sweden, Denmark & Norway |
Cohort study |
Almost 150,000 |
35-84 |
Both male and female |
|
(13) |
China |
Randomized Controlled Trial |
120 |
Not specified |
Not specified |
|
(14) |
China |
Meta-analysis |
254,329 kidney cancer patients |
Not specified |
Not specified |
|
(15) |
Canada |
Cohort study |
1,034 |
63 years (diabetics), 58 years
(non-diabetics) |
Both male and female |
|
(16) |
China |
Meta-analysis |
2,089 patients across 8 studies |
59-67 |
Both male and female |
|
(17) |
Canada |
Retrospective cohort study |
158 |
60.4 years (non-metformin users),
67.3 years (metformin users) |
Both male and female |
|
(18) |
Taiwan |
Retrospective cohort study |
247,252 patients with T2D |
≥40 years |
Both male and female |
|
(19) |
United Kingdom |
Case-control |
24,544 |
<90 |
Male and female |
|
(20) |
China |
Experimental study (in vitro &
in vivo) |
Not applicable |
N/A |
N/A |
|
(21) |
Taiwan |
Retrospective cohort study |
725,316 patients with T2D |
>20 years |
Both male and female |
Risk of bias assessment
The risk of bias assessment (Figure 2) revealed
variability across different domains among the included studies. Selection bias
(D1) was identified as a high risk in 4 out of 10 studies, indicating potential
concerns regarding the representativeness of study populations. Confounding
variables (D2) were generally well controlled, with all studies showing a low
risk in this domain. Measurement of exposure (D3) was consistently rated as low
risk across all studies, enhancing the reliability of exposure assessment.
Blinding of outcome assessment (D4)
remained unclear in 5 studies, suggesting potential detection bias. Incomplete
outcome data (D5) was rated as low risk in all studies, indicating minimal
concerns regarding attrition bias. Selective outcome reporting (D6) was marked
as not applicable in every study, reducing the likelihood of reporting bias.
Overall, while some studies exhibited a
high risk of bias in participant selection and unclear blinding of outcome
assessment, most maintained a moderate to low risk across key domains. These
findings highlight the need for cautious interpretation of the evidence in this
systematic review.
Study Quality Assessment
The
methodological quality of the included observational studies was assessed using
the Newcastle-Ottawa Scale (NOS). Of the six studies eligible for NOS scoring,
three were rated as high quality and three as moderate. Limitations commonly
involved confounding, short follow-up, and lack of adherence data.
Non-observational studies were narratively appraised due to incompatibility
with NOS scoring. A summary of quality appraisal is provided in (Table 4).
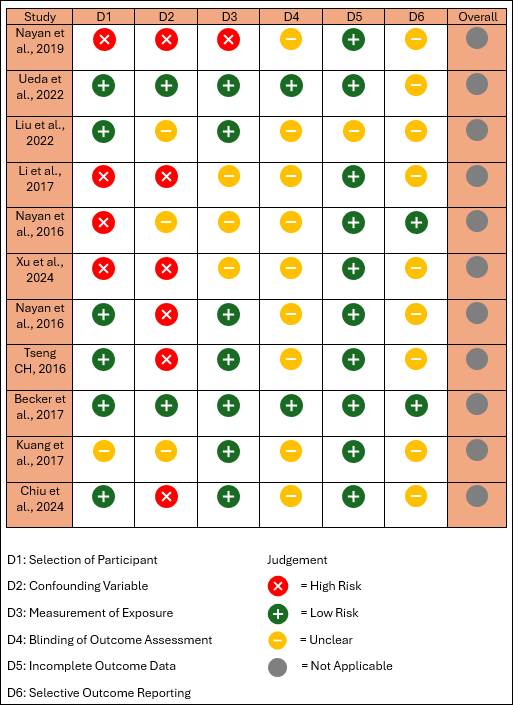
Figure 2. Risk of bias assessment
among studies
This table (Table
4) summarizes the methodological quality of the included studies based on the
Newcastle-Ottawa Scale (NOS) for cohort and case-control studies. Scores range
from 0 to 9 stars, assessing three domains: selection (max 4), comparability (max
2), and outcome/exposure (max 3). Studies were categorized as high quality
(7–9), moderate quality (4–6), or low quality (0–3). Studies with designs not
compatible with NOS (e.g., randomized controlled trials, meta-analyses,
experimental studies) were narratively appraised and marked as "Not
rated." Study-specific limitations, as reported by the original authors or
identified during review, are also noted.
Table 4.. Quality Appraisal of Included Studies Using the Newcastle-Ottawa Scale
(NOS).
|
References |
NOS Score (Out of 9) |
Quality |
Limitations |
|
(12) |
8 |
High |
Unmeasured/residual confounding; outcome
misclassification |
|
(15) |
7 |
High |
Short follow-up: diabetes status at surgery |
|
(17) |
6 |
Moderate |
Small sample; no adherence tracking; no glycemic
control adjustment |
|
(18) |
7 |
High |
No histological confirmation, misclassification;
lifestyle data missing |
|
(19) |
6 |
Moderate |
Misclassification; confounding; missing BMI |
|
(21) |
6 |
Moderate |
Short follow-up, confounding, lack of lab data |
|
(13) |
N/A |
Not Rated |
Small sample, short follow-up, demographic gaps |
|
(11) |
N/A |
Not Rated |
No RCTs; selection bias; exposure definition
variability |
|
(14) |
N/A |
Not Rated |
Observational data only; heterogeneity; no RCTs |
|
(16) |
N/A |
Not Rated |
Small samples, misclassification, observational
studies |
|
(20) |
N/A |
Not Rated |
Preclinical model; lacks mechanistic clarity;
off-target effects |
Strength of Evidence
Among the
included studies, only one was a randomized controlled trial (13), which is
considered the highest level of evidence but was limited by small sample size
and short follow-up. Most studies (12,15,17,18,19,21) were observational cohort
or case-control designs, providing moderate evidence but prone to bias and
confounding. Three studies were meta-analyses (11,14,16), which provide
synthesized evidence but are limited by the quality of included studies. One
study (20) was preclinical, offering mechanistic insights but lacking direct
clinical applicability.
Summary of Drug-specific Outcomes
Table 5 provides a brief overview
summarizing the outcomes of studies according to antidiabetic drug class, using
the reference serial numbers from included studies.
Metformin use did not show a consistent
protective effect on survival outcomes
Table 5. Association between Antidiabetic drugs and renal cancer.
Influencing Factors for Renal Cancer in the Context of Diabetes
Management
A variety of clinical, demographic,
lifestyle, and methodological factors were identified as influencing the
relationship between antidiabetic medications and renal cancer outcomes across
the included studies. Several studies emphasized that the duration and dosage
of metformin use significantly impacted renal cell carcinoma (RCC) risk and
progression (11,16,18,19). The stage of kidney cancer, particularly whether
localized or metastatic, was a consistent determinant of treatment outcomes
(11,14,16,17). In surgical cohorts, factors such as nephrectomy status,
surgical approach (radical vs. partial), and tumor histology were noted to
modulate associations between diabetes treatment and cancer prognosis (15,17).
Patient characteristics—including age,
gender, and existing comorbidities such as hypertension, nephropathy, and
urinary tract disorders—were repeatedly shown to influence study outcomes
(17,18,19,21). Additionally, lifestyle variables such as body mass index (BMI),
smoking, and alcohol use were identified as potential confounders or effect
modifiers in multiple analyses (12,19). Several studies also explored the role
of concurrent antidiabetic medications, suggesting that combined regimens or
changes in treatment (e.g., switching from GLP-1 receptor agonists to SGLT2
inhibitors) could affect risk estimations due to exposure misclassification
(12,18,19).
Importantly, mechanistic studies and
preclinical evidence revealed that SGLT2 expression in RCC cells and their
sensitivity to SGLT2 inhibition may underlie potential protective effects
observed with these drugs (20). Variables such as tumor microenvironment and
duration of SGLT2 inhibitor exposure were highlighted in these experimental
models.
A randomized controlled trial also
emphasized the benefit of comprehensive interventions, including metformin
combined with intensive exercise and dietary modifications, suggesting that
therapeutic outcomes may be enhanced when pharmacological treatment is
integrated with lifestyle changes (13). Methodological approaches—such as
propensity score adjustment, Cox proportional hazards modeling, and weighted
analyses—further shaped study findings by addressing confounding and bias
(12,15,19).
Overall, the observed associations
between antidiabetic drug use and renal cancer outcomes appear to be influenced
by a complex interplay of drug-related, patient-related, and methodological
factors, underscoring the need for cautious interpretation and tailored
analysis in future research.
Discussion
The findings in this systematic review reveal mixed results
regarding the role of antidiabetic medications in kidney cancer outcomes.
Metformin use was associated with improved survival outcomes in some studies
(14), but others reported no significant impact (11,16,17). This inconsistency
aligns with earlier systematic reviews that also found inconclusive evidence
for metformin’s protective effects on renal cell carcinoma (RCC), largely due
to heterogeneous populations, varying study designs, and lack of randomized
controlled trials. Our review adds to the existing body of evidence by
incorporating a broader range of studies, including preclinical data and
real-world cohorts, which provides a more comprehensive picture but also
amplifies the complexity of interpretation.
A key finding was the variability in results across studies, which
can be attributed to differences in exposure definitions (e.g., duration or
dosage of metformin), study populations, follow-up times, and adjustment for
confounders. For instance, the strongest protective effect was reported in a
large retrospective cohort from Taiwan, where a dose-response relationship with
reduced RCC risk was observed (18). However, such observational studies are
inherently prone to residual confounding and misclassification bias, limiting
the ability to draw causal conclusions.
In contrast, SGLT2 inhibitors did not appear to increase RCC risk
(12) and even demonstrated potential protective effects. A large cohort study
(21) showed a significantly lower incidence of RCC among SGLT2 inhibitor users.
Moreover, preclinical evidence from experimental studies supports a
biologically plausible anticancer effect of SGLT2 inhibitors (20), potentially
mediated through inhibition of glucose uptake in tumor cells and modulation of
inflammatory or metabolic pathways. While encouraging, these findings need to
be validated through well-designed clinical trials.
Discrepancies across studies may also be explained by differences
in cancer detection practices, especially in the early treatment phases. For
example, study (12) noted a spike in cancer diagnoses within the first year of
SGLT2 inhibitor use, likely due to detection bias or accelerated presentation
of pre-existing disease. Such early-phase confounding underscores the need for
cautious interpretation of short-term risk elevations.
Additionally, several studies emphasized the importance of
equitable cancer care for diabetic patients. Study (15) advocated for
consistent oncologic management regardless of diabetes status, addressing
concerns about therapeutic nihilism in this subgroup. The lack of demographic,
behavioral, or laboratory data in many studies (e.g., 13, 21) further
complicates interpretation and underscores the need for more granular
real-world datasets.
This review also highlights key limitations in the current evidence
base. The absence of randomized controlled trials (with the exception of a
small study with limited follow-up) (13) constrains the ability to infer
causality. Most included studies were observational and subject to biases such
as selection, immortal time, and outcome misclassification. Heterogeneity in
study design, population characteristics, exposure definitions, and endpoints
limits comparability across findings. Additionally, the lack of standardized
reporting for covariates like BMI, smoking, glycemic control, and comorbidities
undermines internal validity.
Nonetheless, the collective evidence suggests a promising but still
unconfirmed therapeutic potential of certain antidiabetic
medications—especially metformin and SGLT2 inhibitors—in reducing RCC risk or
improving outcomes. Future research should prioritize long-term, multicenter
randomized trials (13,14,16–18,20,21), mechanistic investigations (20), and
studies including diverse populations and robust behavioral/lifestyle profiling
(13,21).
Genetic and Epigenetic Mechanisms Underlying the Observed
Associations
The potential link between antidiabetic medications and renal
cancer outcomes may be partly explained by underlying genetic and epigenetic
mechanisms, particularly those influencing tumor metabolism and progression.
Renal cell carcinoma (RCC) is a genetically heterogeneous disease, often
characterized by mutations in the VHL gene, as well as alterations in chromatin
remodeling genes (e.g., PBRM1, BAP1, SETD2), which affect
tumor suppressor functions and metabolic regulation.
Antidiabetic drugs may modulate some of these pathways indirectly.
For example, metformin, via AMPK activation, suppresses mTOR signaling and may
influence gene expression patterns through epigenetic modulation, including
histone acetylation and methylation. Several studies suggest that metformin can
downregulate oncogenes or upregulate tumor suppressors via altered chromatin
accessibility or DNA methylation. These effects could contribute to reduced
tumor growth or enhanced apoptosis, especially in cancers with dysregulated
mTOR or PI3K-Akt pathways (14,16).
In preclinical models, SGLT2 inhibitors have shown anticancer
effects on RCC cells by interfering with glucose uptake and metabolism (20).
These metabolic alterations can influence gene expression and microRNA (miRNA)
profiles. For instance, SGLT2 inhibition has been associated with modulation of
HIF-1α signaling — a pathway already dysregulated in RCC due to VHL
mutations. Altered glucose handling may also impact histone acetylation status,
leading to changes in tumor cell proliferation and survival.
Although most human studies in this review did not explicitly
examine genetic or epigenetic endpoints, the observed heterogeneity in outcomes
may partially reflect inter-individual genetic variability. Differences in drug
metabolism genes (e.g., OCT1, SLC22A1) could influence metformin
uptake and effectiveness in renal tissues.
Future research should include biomarker stratification and genomic
profiling to better understand the interactions between antidiabetic therapy
and RCC risk. Integrating omics data—such as gene expression, methylation
patterns, and miRNA profiles—into longitudinal cohort studies or clinical
trials may clarify these complex mechanisms and identify subpopulations most
likely to benefit from such therapies. Recommendations from the included studies with their key insights are
given in the table below (Table 6).
Table 6. Key recommendations of
selected studies.
|
References |
Recommendations |
Key Insights |
|
(12) |
SGLT2 inhibitors might elevate the short-term risk of certain
outcomes, possibly due to their influence on existing cancers or increased
early detection. A significant rise in risk was seen within the first year. |
Early risk spike may be due to detection bias or underlying
disease acceleration. |
|
(13) |
Larger, long-term studies are needed; include detailed
demographic and health data. Promote adherence to lifestyle changes. |
Need for robust methodology, lifestyle impact, and longitudinal
evidence. |
|
(14) |
Additional well-designed studies are needed to assess metformin’s
impact on kidney cancer survival in diabetics. |
Current evidence on metformin’s survival benefit in kidney cancer
is inconclusive. |
|
(15) |
Diabetic patients should receive the same standard of care and
monitoring as non-diabetic individuals. |
Importance of equitable clinical management. |
|
(16) |
Future research on metformin and RCC should use large,
multicenter studies with strong clinical designs. |
Need for more generalizable and methodologically rigorous
studies. |
|
(17) |
Population-level studies are needed to further explore
metformin’s role in kidney cancer. |
Emphasis on broader epidemiological validation. |
|
(18) |
Randomized clinical trials are essential to confirm metformin’s
protective role against kidney cancer. |
Strong evidence can only come from controlled trials. |
|
(19) |
Clinical trials should test dapagliflozin’s safety and efficacy
in RCC. Study molecular mechanisms and explore combined therapies. Long-term
outcomes and side effects should be assessed. |
Multifaceted research agenda on dapagliflozin’s role in RCC
needed. |
|
(20) |
More RCTs with longer follow-up are needed for SGLT2 inhibitors.
Collect detailed patient behavior and lab data. Study vulnerable populations. |
Tailored, long-term evidence needed to understand SGLT2
inhibitors' role across subgroups. |
Summary of Recommendations
- Early Risk
Concerns
- Robust Study
Designs Needed
- Equal Clinical
Management
- Demographic and
Behavioral Data
- Mechanistic and
Combination Therapy Research
- Personalized
Care: Clinicians should be vigilant when initiating SGLT2 inhibitors,
particularly during the first year, and tailor cancer screening and follow-up
accordingly.
- Therapeutic
Potential: Metformin and SGLT2 inhibitors hold promise as adjuncts in managing
renal cell carcinoma, but they require more definitive evidence before clinical
adoption.
- Holistic
Management: Diabetes status should not preclude patients from receiving optimal
cancer care; equity in clinical monitoring and treatment is essential.
- Evidence-Based
Guidelines: Results highlight the need to update treatment protocols based on
evolving evidence, especially regarding newer antidiabetic agents with
potential oncologic implications.
Conclusion
This systematic review highlights the
potential role of metformin and SGLT2 inhibitors in kidney cancer outcomes.
While metformin may provide survival benefits in some patient populations,
findings remain inconsistent across studies. SGLT2 inhibitors appear to have a
neutral to beneficial effect on kidney cancer risk, with emerging evidence
suggesting anticancer properties. Given the limitations in study design and
potential confounding factors, further large-scale, high-quality studies are
needed to establish definitive conclusions regarding the role of these
antidiabetic medications in kidney cancer treatment and prevention.
Author contribution
SN developed the methodology and wrote the
methodology section. SN also conducted data extraction using a predesigned
Excel spreadsheet, capturing key study details, including study design, patient
population, type of antidiabetic medications used, renal cancer outcomes, and
major findings. Additionally, SN oversaw the entire review process and
coordinated the writing of the manuscript. MH
independently verified 50% of the extracted data to ensure accuracy and
consistency. MH also wrote the results section, contributed to the final review
of the manuscript, played a role in developing the study design, and assisted
in refining the methodology section. AN contributed
to refining the search strategy, participated in the full-text review process,
and assisted in synthesizing the extracted data. AN also built the tables and
diagrams for the manuscript and helped review the methodology section. AH independently conducted the title
and abstract screening using Rayyan software, ensuring the initial selection of
studies. AH also conducted the full-text review for studies meeting the
inclusion criteria and wrote the discussion section. TS independently verified 50% of the extracted data alongside MA to
enhance data accuracy. TS also contributed to refining the study methodology
and participated in manuscript revisions. NN
wrote the introduction section and assisted in optimizing the search
strategy. NN also played a role in screening full-text articles and contributed
to drafting and reviewing the discussion section. AT independently conducted the title and abstract screening using
Rayyan software, ensuring the initial selection of studies. AT also wrote the
conclusion section and participated in discussions regarding study inclusion
and exclusion criteria. NK contributed
to writing the discussion section and provided critical revisions to improve
clarity and coherence. NK also participated in reviewing the final manuscript
to ensure consistency and accuracy. RA played
a role in the quality assessment of included studies and assisted in
synthesizing the extracted data. RA also contributed to reviewing the
discussion and conclusion sections to ensure alignment with the study
objectives. All authors contributed to the conception and design of the study,
provided input on data interpretation, and participated in manuscript
revisions. All authors approved the final version before submission
Conflict of interest
The author declares no conflict of interest associated with this
paper.
Funding
There is no funding.
References
1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S,
Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and
projections for 2030 and 2045: Results from the International Diabetes
Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract.
2019 Nov 1;157:107843.
2. Ardha PW, Khairun BN. Empat Pilar Penatalaksanaan Pasien Diabetes Mellitus Tipe 2.
2015;4(9):undefined-undefined.
3. Nishimura R, Kato H, Kisanuki K, Oh A, Hiroi S, Onishi Y, et al. Treatment
patterns, persistence and adherence rates in patients with type 2 diabetes
mellitus in Japan: A claims-based cohort study. BMJ Open. 2019 Mar 1;9(3).
4. Khan MAB, Hashim MJ,
King JK, Govender RD, Mustafa H, Kaabi J Al.
Epidemiology of Type 2 Diabetes – Global Burden of Disease and Forecasted
Trends. J Epidemiol Glob Health. 2020 Mar 1;10(1):107.
5. Graff RE, Sanchez A,
Tobias DK, Rodríguez D, Barrisford GW, Blute ML, et
al. Type 2 diabetes in relation to the risk of renal cell carcinoma among men
and women in two large prospective cohort studies. Diabetes Care. 2018 Jul 1;41(7):1432–7.
6. Xu CX, Zhu HH, Zhu YM.
Diabetes and cancer: Associations, mechanisms, and implications for medical
practice. World J Diabetes 2014;5(3):372.
7. Coggan JL, Tan A, Kuzel
TM. Renal Cancer. Cancer Consult: Expertise in Clinical Practice, Volume 1:
Solid Tumors and Supportive Care. 2024;1:361–73.
8. Tseng CH. Use of
metformin and risk of kidney cancer in patients with type 2 diabetes. Eur J Cancer. 2016 Jan 1;52:19–25.
9. Becker C, Jick SS, Meier CR, Bodmer M. Metformin and the risk of
renal cell carcinoma: a case-control analysis. Eur J
Cancer Prev. 2017 May;26(3):257-262.
10. Liu YC, Nguyen PA,
Humayun A, Chien SC, Yang HC, Asdary RN, Syed-Abdul
S, Hsu MH, Moldovan M, Yen Y, Li YJ, Jian WS, Iqbal U. Does long-term use of
antidiabetic drugs changes cancer risk? Medicine (Baltimore). 2019 Oct;98(40):e17461.
11. Nayan M, Punjani N,
Juurlink DN, Finelli A, Austin PC, Kulkarni GS, Uleryk
E, Hamilton RJ. Metformin Use and Kidney Cancer Survival Outcomes: A Systematic
Review and Meta-Analysis. Am J Clin Oncol. 2019 Mar;42(3):275-284.
12. Ueda P, Svanström H, Hviid A, Eliasson B, Svensson AM, Franzén S, Gudbjörnsdottir S, Hveem K, Jonasson C, Wintzell
V, Melbye M, Pasternak B. Sodium-Glucose Cotransporter 2 Inhibitors and Risk of
Bladder and Renal Cancer: Scandinavian Cohort Study. Diabetes Care. 2022 May
1;45(5):e93-e96.
13. Liu Y, Meng LL, Li JW,
Jin YS, An RH. A Randomized Study on the Effect of Metformin Combined with
Intensive-Exercise Diet Therapy on Glucose and Lipid Metabolism and Islet
Function in Patients with Renal Cell Carcinoma and Diabetes. Dis Markers. 2022
Jul 15;2022:7383745.
14. Li Y, Hu L, Xia Q, Yuan
Y, Mi Y. The impact of metformin use on survival in
kidney cancer patients with diabetes: a meta-analysis. Int Urol
Nephrol. 2017 Jun;49(6):975-981.
15. Nayan M, Finelli A,
Jewett MA, Juurlink DN, Austin PC, Kulkarni GS, Hamilton RJ. Diabetes and
kidney cancer outcomes: a propensity score analysis. Endocrine. 2017
Feb;55(2):470-477.
16. Xu K, Ying Y.
Meta-Analysis of Metformin on Recurrence Risk and Long-Term Survival in
Patients with Diabetes and Renal Cell Carcinoma. Altern Ther Health Med. 2024
Apr;30(4):60–5.
17. Nayan M, Finelli A,
Jewett MA, Juurlink DN, Austin PC, Kulkarni GS, Hamilton RJ. Metformin Use and
Kidney Cancer Outcomes in Patients With Diabetes: A
Propensity Score Analysis. Clin Genitourin Cancer.
2017 Apr;15(2):300-305.
18. Tseng CH. Use of
metformin and risk of kidney cancer in patients with type 2 diabetes. Eur J Cancer. 2016 Jan;52:19-25.
19. Becker C, Jick SS, Meier CR, Bodmer M. Metformin and the risk of
renal cell carcinoma: a case-control analysis. Eur J
Cancer Prev. 2017 May;26(3):257-262.
20. Kuang H, Liao L, Chen H,
Kang Q, Shu X, Wang Y. Therapeutic Effect of Sodium Glucose Co-Transporter 2
Inhibitor Dapagliflozin on Renal Cell Carcinoma. Med Sci Monit. 2017 Aug 1;23:3737-3745.
21. Chiu CH, Wang WY, Chen
HY, Liao PL, Jong GP, Yang TY. Decreased risk of renal cell carcinoma in
patients with type 2 diabetes treated with sodium glucose cotransporter-2
inhibitors. Cancer Sci. 2024 Jun;115(6):2059-2066.