Introduction
Tenosynovial giant cell tumour (TGCT) is a rare mesenchymal¬ tumour that affects
joints and tendon sheaths. The diffuse-type TGCT has a high risk of relapse
following surgical resection of the tumour (1).
The classification of it can be categorised
into ‘nodular’ and ‘diffuse’ TGCT depending on the imaging findings of the
pathology. TGCT can be either benign or malignant – the likely incidence of
malignant TGCT is lower than that of benign TGCT at less than 1 per million per
year, with a mortality rate of around 30% and a metastatic rate of around 50%
(1). Estimates suggest that there are approximately 10 per million
persons/year for the localised type, while the diffuse type is about 4 per
million persons/year (2). Burton et al. highlights that little is known on the
burden of disease and that the common presentation of TGCT is benign (3). This
illustrates the rarity of the above condition.
A Japanese-authored article published by
Takeuchi et al., illustrated case reports of two cases of TGCT, with one having
an ocular cancer (Choroidal Melanoma), while the other was associated with
multiple type 1 neurofibromatosis (4). This is important as choroidal melanoma
is the second most common site of ten malignant melanoma sites in the body (5).
An article published in 2021 by Italian authors highlighted that pure muscle tenosynovial giant cell tumour mimics a metastasis in a
patient with melanoma. In this study, a 50-year-old female with a diagnosis of
malignant melanoma presented for a routine scan and an intense FDG focal uptake
corresponding to peri-trochanteric medial part of right iliopsoas muscle was
discovered (6). Once biopsied, the final diagnosis was derived. This is of
importance as the TGCT lesions may reproduce a malignant appearance on FDG-PET.
Hence, TGCT may be under-diagnosed as patient’s may be diagnosed as ‘metastatic
melanoma’ rather than biopsying the lesion which may
show that the histology is actually a second primary lesion. Furthermore, a
recent article published in 2025 by Patel et al. has documented that second
primary cancers does occur in the setting of melanoma. His focus looked at a
more common cancer (colo-rectal cancer) and
associations between the two. In this study, it was confirmed that it can be
influenced by various factors e.g. biological, lifestyle, genetic factors (7).
There were no other studies which are
published (to our knowledge) of a patient diagnosed with malignant melanoma who
also has malignant TGCTThere were no other studies
which are published (to our knowledge) of a patient diagnosed with malignant
melanoma who also has malignant TGCT.
Case presentation
Mr X, an 87-year-old South African male
with a background history of malignant melanoma (excised in 1982), in
remission; presented with shortness of breath and a cough. He was a non-smoker
and of sober habits and had a history of a pacemaker inserted for a
tachyarrhythmia. He had no other comorbidities. An initial baseline chest X-Ray
(CXR) performed on 26 May 2024 revealed a right pleural effusion (Figure 1).
This caused the shortness of breath in the patient and was considered treatable
as the fluid could be drained, which would lead to symptomatic relief.
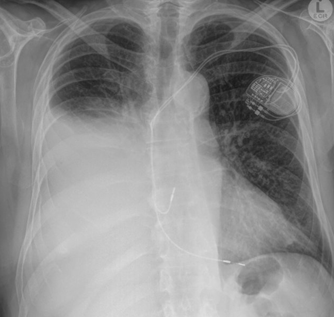
Figure 1. CXR performed on 26 May 2024.
A CT scan was thereafter performed which
demonstrated multiple pleural-based nodules and masses,
the largest of which was adjacent to right 6th and 7th ribs and measured 3.3cm x 4.7cm (figure 2)
The second mass was noted adjacent to the right side
aspect of T8 and T9 vertebral bodies and measured 2.0 x 2.6cm (figure 3).
There was also associated pleural
thickening in the right costophrenic angle with an associated pleural based
lesion measuring 2.0 x1.9cm as well as a right hilar node which was 1.4cm (figure 4).
There was also a right large pleural
effusion.
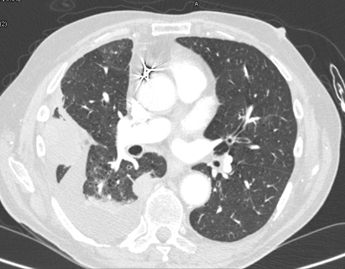
Figure 2. CT scan: multiple pleural-based nodules and masses,
the largest of which was adjacent to the right 6th and 7th ribs.
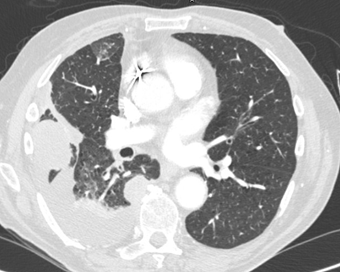
Figure 3. The second mass was noted adjacent to the
right side aspect of T8 and T9 vertebral bodies.
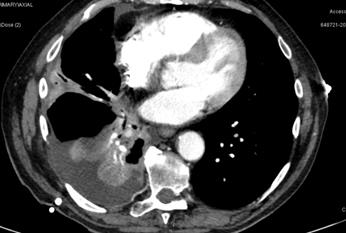
Figure 4. Associated pleural thickening in the right
costo-phrenic angle with an associated pleural-based
lesion measuring 2.0 x 1.9 cm as well as
a right hilar node which was 1.4cm.
The patient was referred to the
cardio-thoracic surgeon and had a pleurocentesis
performed. After the drainage, the newer CXR demonstrated improvement in the
pleural effusion and unmasked a mass in the right lung which was previously
hidden by the fluid. There was right basal atelectasis and a small residual
right pleural effusion with a peripheral right mid-zone 51 mm nodule (See
Figure 5).
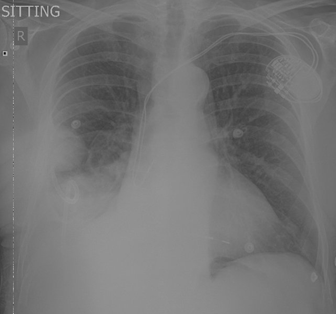
Figure 5. CXR post pleural tap.
Following this, Mr X had a pleural biopsy
via a video-assisted thoracoscopic surgery (VATS) procedure and had a right
pleural drain inserted. The specimens were reviewed by a local histopathologist
at a private pathology laboratory and the patient was referred to the Oncology
unit for further work-up and management whilst awaiting histopathology results.
No PET scan was performed as the nearest
PET scanner is over 250km away from the Oncology Practice. With regard to
tumour markers (S100), the patient’s older records from his melanoma were not
available from the 1980s or 1990s. No S100 marker was done as the diagnosis was
not a metastatic lesion from the melanoma.
Immuno-histo-chemistry
Histology was done from the biopsy
specimens:
Initially, considering the clinical history
of previous malignant melanoma, a tentative diagnosis of metastatic malignant
melanoma with osteoclast-like giant cells was favoured (Table 1).
Table 1. Immuno-histochemical stains were done and revealed.
|
Marker |
Result |
Interpretation |
|
SOX-10 |
Negative |
Rules out melanocytic or neural crest
origin (e.g. melanoma, schwannoma). |
|
PRAME |
Positive in isolated cells |
PRAME is a non-specific marker; can be
seen in both benign and malignant settings. Not diagnostic. |
|
Calretinin |
Patchy Positive |
Suggests presence of mesothelial or
reactive mesothelial cells. Not specific to tumour. |
|
OSCAR |
Positive in scattered cells |
OSCAR (pan-cytokeratin) suggests focal
epithelial features, but not a dominant feature. |
|
BAP1 |
Positive (retained expression) |
Nuclear retention of BAP1 argues against
malignant mesothelioma. |
|
CD45 |
Positive in normal lymphocytes |
Confirms presence of background lymphoid
cells; tumour is not of lymphoid origin. |
|
HMB45 |
Negative |
Rules out melanoma and other PEComas. |
|
Vimentin |
Strongly and diffusely positive |
Suggests mesenchymal origin, which aligns
with many soft tissue tumours, including TGCT. |
|
MUM-1 |
Negative |
MUM-1 negativity excludes significant
plasma cell or lymphoid differentiation. |
|
WT-1 |
Patchy positive |
Highlights mesothelial or entrapped
mesothelial cells; not specific for the tumour itself. |
Key: SOX-10: transcription factor that is
part of a gene family with a DNA-binding HMG box domain; PRAME: preferentially
expressed antigen in melanoma; BAP1: OSCAR: Osteoclast-associated receptor;
BRCA1-associated protein; CD45: Cluster of Differentiation 45; HMB45: Human
Melanoma Black 45; MUM-1: multiple myeloma oncogene-1; WT-1: Wilms's Tumour
1; Due to the complexity of the case as
described in the Immuno-histochemistry stains seen (see Table 1), it was sent
to Cape Town for a second histopathological opinion, where a professor in
histopathology/cytopathology reviewed the diagnosis. This caused a delay in commencing treatment
of around 3 weeks for the patient.
Microscopy showed the following (verbatim):
“multiple cellular fragments of neoplastic tissue. Some of the fragments have
overlying surface fibrin. In some of the fragments collagenous areas are
present. The tumour comprised both solid and pseudo-alveolar areas. The solid
areas comprised diffuse groups of tumour cells. The tumour cells were
mononuclear with eosinophilic cytoplasm and slightly conspicuous nucleoli.
There were admixed macrophages. In addition, there were unevenly distributed osteoclast-type
giant cells. In other areas, the tumour cells were discohesive,
forming pseudo-alveolar structures. Within some of these areas, blood lakes
were present which were not lined by endothelium. Tumour cells were seen
floating in the blood lakes. In these blood lakes, hemosiderin-laden
macrophages were present. Some of the tumour cells are surrounded by fibrin. In
some of the more collagenous areas, these cells appear more spindled rather
than epithelioid. Mitotic activity is present. Melanin pigment was not appreciated”.
From Table 2, the
final diagnosis stated from the pleural biopsy was ‘Extra-Articular Tenosynovial Giant Cell Tumour, Diffuse Type’.
Table 2. Repeat immunohistochemistry revealed the following findings.
|
Marker / Stain |
Result |
Interpretation |
|
OSCAR |
Positive
at periphery, negative in most cells |
Suggests
epithelial origin in periphery; not significant overall |
|
CD45 |
Scattered
positive cells |
Scattered
lymphoid cells; not a lymphoid neoplasm |
|
Calretinin |
Scattered
cytoplasmic positivity in mesothelial cells |
Highlights
entrapped/reactive mesothelial cells |
|
BAP-1 |
Diffuse
nuclear retention |
No loss;
argues against mesothelioma |
|
WT-1 |
Weak
nuclear positivity in many cells |
Highlights
mesothelial cells; not tumour-specific |
|
Vimentin |
Diffuse
positive staining |
Consistent
with mesenchymal origin |
|
HMB45 |
Negative |
Rules out
melanoma |
|
SOX-10 |
Negative |
Rules out
neural crest tumours (e.g., melanoma, schwannoma) |
|
PRAME |
Some
nuclear positivity |
Non-specific;
seen in some neoplastic and benign processes |
|
MUM-1 |
Scattered
positivity in plasma cells |
Reflects
background immune cells |
|
Melan A |
Negative |
Rules out
melanoma |
|
CD68 |
Diffuse
positive; highlights multinucleated giant cells |
Typical
for histiocytic origin in tenosynovial giant cell
tumour |
|
p63 |
Interpreted
as negative |
Argues
against epithelial or myoepithelial differentiation |
|
AE1/AE3 |
Positive
in some cells, similar to OSCAR |
Focal
epithelial marker expression; non-specific |
|
ERG |
Positive
in blood vessels only |
Normal
vascular staining; not tumour-specific |
|
Desmin |
Scattered
positive tumour cells |
Suggests
some myoid differentiation; may be non-specific |
|
Perl’s
Prussian Blue |
Positive
in hemosiderophages and some tumour cells |
Indicates
iron deposition; common in tenosynovial giant cell
tumour |
Key: SOX-10: transcription factor that is
part of a gene family with a DNA-binding HMG box domain; PRAME: preferentially
expressed antigen in melanoma; BAP1: OSCAR: Osteoclast-associated receptor;
BRCA1-associated protein; CD45: Cluster of Differentiation 45; HMB45: Human
Melanoma Black 45; MUM-1: multiple myeloma oncogene-1; WT-1: Wilms's Tumour
1; PRAME: PReferentially
expressed Antigen in Melanoma; CD68: Cluster of Differentiation 68; AE1/AE3:
Anion Exchanger 1/ Anion Exchanger 3; ERG: ETS-related gene.
Oncology
Mr X presented to
Oncology on 5 June 2024, with a history of shortness of breath and cough. A
working diagnosis of recurrent metastatic malignant melanoma was favoured at
the time, based on histopathological morphological findings and the previous
clinical history of melanoma. He had an ECOG performance status of 2 and was
planned for systemic therapy for metastatic malignant melanoma.
After review of
the updated histopathology report and the diagnosis of TGCT, Imatinib was
considered as part of the therapeutic options as well as radiotherapy to the
large thoracic masses for symptomatic control.
Other treatment options were not available and Denosumab would have been
a good choice, but Mr X had more visceral disease than skeletal involvement.
From the initial presentation, his condition declined.
On 18 July 2024, a repeat CT scan was
performed. It showed significant disease progression in the necrotic pleural
based mass along the right hemithorax with right lower zone empyema. There was
also mediastinum and right hilum lymphadenopathy present.
Mr X also had a CT scan of the brain as he
was confused to exclude brain metastases which came back negative.
The patient
ultimately succumbed to his illness and passed away on 22 July 2024.
Discussion
A South African
article published by Tontu et al. in 2024, described
Denosumab in managing TGCT in a 21-year-old female (8). Unfortunately, in our
patient, this drug was not available. Furthermore, in Mr X, it was initially
thought that the lung lesion was a metastatic lesion, rather than second
primary lesion. It has been found that a biopsy of a suspicious lesion can
confirm if there is a relapse of the tumour or exclude the second primary
tumour in metastatic lesions (9). We postulate that biopsies of suspected
(likely) metastatic lesions may reveal that some of these lesions may actually
be a second primary lesion. This could impact management as a different regimen
of drugs may be considered. An article published by Zheng et al. supports this
as it was found that around 25% of patients diagnosed with cancer have a second
primary malignancy (10). Another interesting finding from Mr X was the
association with melanoma. This case may support a rare co-occurrence, although
causality is unproven between TGCT and melanoma, as this would be the second
case documented (to our knowledge) of such an association in such a rare
condition.
Immunohistochemistry
assisted greatly with the diagnosis. The initial comment from the
histopathologists mentioned that “Vimentin positivity may be seen in melanoma,
however, the negative SOX-10, HMB45 and only patchy positive PRAME, demand that
other possibilities be considered. Vimentin positivity is seen in a host of
other tumours including mesothelioma (excluded with calretinin and BAP1
stains), sarcomas and giant cell tumours, the latter of which may be considered
in this case given the morphology of the tumour, except the tumour site is
quite unusual” (11). Considering this and the second opinion of an expert
immuno-histopathologist, the diagnosis was male.
The “best
clinical management of tenosynovial giant cell tumour
(TGCT): A consensus paper from the community of experts” published in 2022 was
designed to assist with helping clinicians with the management of TGCT (1).
Colony-stimulating factor 1 receptor (CSF1R) inhibitors are effective for
symptomatic benefit and improves the quality of life of patients with TGCT but
is not available in many countries (1). In South Africa, this drug is not
available; however, a drug with the tradename “Turalio”
is available for treatment of TGCT internationally (12). If a patient is diagnosed with TGCT, it is
recommended that they go to expert centres with experienced sarcoma
multidisciplinary treatment team (1). Thereafter a joint decision can be made
about active surveillance vs active treatment, surgery, radiotherapy,
cryotherapy or systemic treatment (1). In South Africa, teams like this exist
in Cape Town; however, given the patient’s advanced stage of disease, he was
not considered for referral for this multidisciplinary team. It is likely that
the delay in commencing treatment also led to the progression of disease in
this patient, highlighting the need for referring patients like this to such teams.
With regards to
clinical decision making, Imatinib was used as it inhibits the tyrosine kinase
activity of the CSF1R, which is an important protein which affects the growth
and proliferation of these tumours. Using this drug would result in the pathway
being blocked, which would lead to shrinkage of the tumour which would improve
symptoms (13). The other drug considered
was Denosumab which is a systemic monoclonal antibody against the Receptor
Activator of Nuclear factor Kappa-B (RANK) ligand, which has been used in
patients with giant cell tumours of the bone (14). Our rationale of using
Imatinib over Denosumab was that Denosumab is used for bone metastasis in most
cases. In the case of Mr X, there was no clear evidence of bone involvement so
Imatinib was favoured as his tumour showed mostly soft tissue (lung parenchyma)
involvement. Of note, there isn’t a clear guideline or consensus on this as the
case is uncommon.
Conclusion
TGCT is a rare condition which may or may
not be associated with melanoma. We recommend that suspected metastatic
melanoma lesions be biopsied to establish or refute this association.
Acknowledgement
Pathcare (private
laboratory) assisted with the Histopathology on this case. We would like to
thank the histopathologists who were involved in producing the pathology reports:
Dr Jaravaza, Dr Sengoatsi
and Prof Govender.
Author contribution
RRC and SK were both involved in writing/editing the manuscript.
Conflict of interest
The author declares no conflict of interest associated with this
paper.
Funding
There is no funding.
Ethical considerations
All information was anonymised to ensure
patient confidentiality. Permission for using anonymised patient data was
granted by Cancercare.
References
1. Stacchiotti
S, Durr HR, Schaefer IM et al. Best clinical management of tenosynovial
giant cell tumour (TGCT): A consensus paper from the
community of experts. Cancer Treat Rev. 2023 Jan; 112:102491.
2. Aboo
FB, Kgagudi PM. Tenosynovial
giant cell tumour: current concepts review and recent
developments focusing on pathogenesis and treatment-directed classification. SA
Orthop J. 2025;24(2):90-93.
3. Burton TM, Ye X, Parker
ED, Bancroft T, Healey J. Burden of Illness Associated with Tenosynovial
Giant Cell Tumours. Clin Ther. 2018 Apr;40(4):593-602.
4. Takeuchi A, Yamamoto N,
Hayashi K et al. Tenosynovial giant cell tumours in unusual locations detected by positron emission
tomography imaging confused with malignant tumours:
report of two cases. BMC Musculoskelet Disord. 2016 Apr 26(17):180.
5. Singh P, Singh A.
Choroidal melanoma. Oman J Ophthalmol. 2012
Jan;5(1):3-9.
6. Arcuri PP, Commisso A,
John M, Cascini GL, Laganà D. Pure muscle tenosynovial giant cell tumour mimicks a metastasis in patient with melanoma. J Clin
Images Med Case Rep. 2021; 2(4):1242.
7. Patil S, Jeyakumar A,
Gopalan V. Melanoma and Colorectal Cancer as Second Primary Cancers: A Scoping
Review of Their Association and the Underlying Biological, Lifestyle, and
Genetic Factors. J Gastrointest Canc. 2025.(56):125.
8. Tontu
NA, Miseer S, Burger H. Denosumab in irresectable
giant cell tumour of the cervical spine. South
African Journal of Oncology. 2024; 8(0), a303.
9. Qu Q, Zong Y, Fei XC et
al. The importance of biopsy in clinically diagnosed metastatic lesions in
patients with breast cancer. World J Surg Oncol. 2014 Apr (10):934.
10. Zheng X, Li X, Wang M et
al. Multidisciplinary Oncology Research Collaborative Group (MORCG). Second
primary malignancies among cancer patients. Ann Transl
Med. 2020 May; 8(10):638.
11. Pathcare Laboratories.
Confidential Histopathology Patient Report. 17 July 2024.
12. Dharmani
C, Fofah O, Fallon M, Rajper
AW, Wooddell M, Salas M. TURALIO® Risk Evaluation and Mitigation Strategy
Program (tREMS): 3-year retrospective hepatic safety
assessment. Future Oncol. 2024;20(33):2559-2564.
13. Cassier PA, Gelderblom H, Stacchiotti S et
al. Efficacy of imatinib mesylate for the treatment of locally advanced and/or
metastatic tenosynovial giant cell tumor/pigmented
villonodular synovitis. Cancer 2012;118:1649-1655.
14. Tontu
NA, Miseer S, Burger H. Denosumab in irresectable
giant cell tumour of the cervical spine. S. Afr. J.
Oncol. 2024; 8(0), a303.