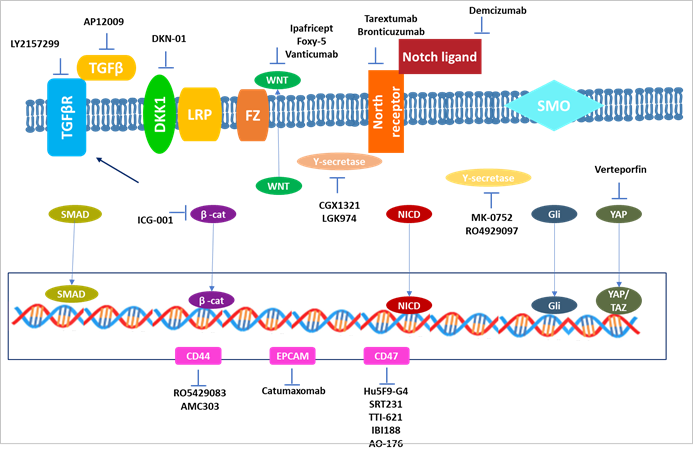
Figure 1. Treatments that target CSCs.
Anti-CSC medicines that target developmental pathways and CSC-associated
surface indicators have been identified.
Investigating the
molecular mechanism of cancer stem cells (CSCs) in treatment of
gastrointestinal cancers
Seyedeh Elham
Norollahi 1, Sogand Vahidi 2, Fatemeh Nejatifar 3*, Ali Akbar Samadani 4,5*
1 Cancer Research Center and Department of Immunology, Semnan
University of Medical Sciences, Semnan, Iran
2 Medical Biology Research Center, Kermanshah University of Medical
Sciences, Kermanshah, Iran
3 Department of Hematology and Oncology, Razi hospital, School of
Medicine, Guilan University of Medical Sciences, Rasht, Iran
4 Department of Basic Medical Sciences, Neyshabur University of
Medical Sciences, Neyshabur, Iran
5 Guilan Road
Trauma Research Center, Guilan University of Medical Sciences, Rasht, Iran
Corresponding Authors: Ali Akbar
Samadani * Email: a_a_hormoz@yahoo.com
Fatemeh Nejatifar *
Email: dr.f.nejatifar@gmail.com
Abstract
Cancer stem cells (CSCs) are involved in tumor formation, drug and
radiation resistance, invasive growth, metastasis, and tumor progression and
are major causes of cancer-related mortality. Gastrointestinal cancers are one
of the most common malignancies and causes of cancer death worldwide. Because
gastrointestinal cancer stem cells are thought to be resistant to common
treatments, new and effective treatments are needed. Cancer stem cells have
been reported in colorectal, esophageal, gastric, liver and pancreatic cancers.
Given that, understanding the formation of cancer stem cells and identifying
control pathways and investigating the molecular mechanism of signaling
involved in these cells and their role in cancer treatment leads to the
development of diagnostic and therapeutic methods in basic and clinical cancer
research. In this study, the functional role and molecular mechanisms of cancer
stem cells in the treatment of gastrointestinal cancers are investigated.
Keywords: Cancer stem cells, Molecular mechanism, Gastrointestinal cancers
Introduction
Gastrointestinal cancer is the development of tumors
from the proximal esophagus to the distal rectum, including cancers of the
liver and bile ducts that extend to the intestinal lumen. Despite advances in
surgery, endoscopy, chemotherapy, and radiation therapy, patients with these
cancers continue to suffer from recurrence and progression. Cancer stem cells
(CSCs) found in heterogeneous tumors are known to be major contributors to
cancer recurrence and progression. CSCs are very important in disease
recurrence because they have properties that make them resistant to
chemotherapy and radiation therapy (1, 2).
Cancer stem cells are responsible for tumor growth,
drug and radiation resistance, invasive growth, metastasis, and tumor
recurrence, which are major causes of cancer-related deaths. Because
gastrointestinal CSCs are thought to be resistant to conventional therapies,
effective and new cancer treatment is necessary (3). CSCs are derived from normal adult normal stem cells, progenitor cells,
and differentiated adult cells. Thus, signal transduction pathways in CSCs that
play an important role in self-renewal are similar to those involved in normal
fetal growth (4).
These pathways include Wnt, Hedgehog, and Notch
signaling in addition to the polycomb group protein pathways. In addition,
growth factors such as fibroblast growth factor, insulin-like growth factor-1,
and TGF ‐ β may also play a role in controlling CSCs. Proinflammatory cytokines
facilitate the production of CSCs, indicating a possible link between cytokines
and inflammation. Hypoxia also plays an important role in regulating
self-renewal in normal cells and CSCs (5-7).
Chemotherapy
and radiotherapy are the main treatments for cancers of the gastrointestinal
tract including the esophagus, stomach, liver, pancreas and rectum.
Unfortunately, there is a recurrence and progression of the disease with these
treatments. The mechanism that CSC performs in the treatment of cancer includes
increased DNA repair, incubation, and drug release and redox capacity. CSCs are
armed with multiple mechanisms to escape conventional cancer treatment, so this
limits treatment options and allows CSCs to cause disease recurrence and
metastasis. Therefore, ideal antitumor therapies should target both
proliferating cancer cells and CSCs. In this regard, induction and
differentiation therapies are targeted to eliminate CSCs (8, 9). Combining several treatments such
as surgery, endoscopy, chemotherapy and radiation therapy may improve survival
in patients with gastrointestinal cancer. However, the effectiveness of these
treatments depends on the cancer status, metastasis, radiation/chemotherapy
resistance, and recurrence, which are thought to be due to CSC. Therefore, new
treatment options for these diseases must be developed (10).
In
this study, we investigated the role and application of cancer stem cells and
the molecular mechanisms of signaling involved in gastrointestinal cancers.
Cancer
stem cell characteristics, tumor heterogeneity, and treatment resistance
Intratumor and intratumor heterogeneity are two types of tumor
heterogeneity. Tumor heterogeneity can be caused by the origin cells. PDAC and
pancreatic neuroendocrine neoplasm, for instance, are two main pancreatic tumor
histological categories. Pancreatic neuroendocrine malignancy is further split
into two types: well enough and inadequately pancreatic neuroendocrine
carcinoma (PanNEC). Different driver genes can display heterogeneity among
PanNEC, PDAC, and PanNET. KRAS, SMAD4, CDKN2A, and TP53 are among the major
driver gene alterations discovered in PDAC. PanNEC has mutations in the KRAS,
TP53, and RB1 genes, whereas PanNET has mutations in the MEN1, DAXX/ATRX, and
mTOR pathway genes, which are completely different from those found in PDAC and
PanNET. In addition, the origins of PDAC, PanNEN, and PanNEC are murky. PDAC
can be caused by intralobular duct precursor cells or acinar cells with
exocrine secretion. PanNETs can come from the -cell lineage, islet cell
precursors, or the -cell lineage. Originating PanNEC cells could be
undifferentiated progenitor cells with stem cell-like features (11).
CSCs are divided into subpopulations with different roles,
developmental processes, and gene expression patterns (12, 13). CSC
populations can be isolated and identified using cell surface markers.
Hematopoietic and embryonic stem cells provide the majority of the indicators.
Nanog, Sox2, Oct4, and c-Myc are among the markers that have been considered
favored stemness markers. Various indicators have been identified to
characterize CSC populations in many cancer types (Table 1); for example, the
combination of CD24 and CD44 markers define a common CSC population for
colorectal cancer, liver cancer, pancreatic cancer, and other cancer types.
Surprisingly, this population also describes the breast cancer mesenchymal-like
CSC population. Furthermore, the expression of most CSC indicators differs
among tumor types and even within the same subtype of patients. CD24, for
example, was shown to be considerably lower in oral squamous cell carcinoma and
considerably larger in pancreatic intraepithelial neoplasia (14).
Due to the obvious absence
of uniformity, indicator, EpCAM-in pure populations, and using various markers
to enrich CSCs optimally could help. Indeed, EpCAM, CD166, and CD44 were more
reliable than CD133 alone as indicators of colorectal cancer.
Table 1. Representative markers of gastrointestinal CSCs.
|
Gastrointestinal cancer |
Factors |
|
Gastric cancer |
CD44+, Lgr5+, CD44+/CD24+, CD133+, CD44+/Snail1+/VIMENTIN+/E-cadherin+,
Snail+, CD44v8-10+, Frizzled7+ |
|
Colorectal cancer |
E-cadherin-,
CD44v2+, CD44v6+, ALDH, CD133+, CD44+/CD24+, CD166+, CD133+/CD44+/ALDH1+ |
|
Esophageal cancer |
CD44+, B7H4+, CD133+/CXCR4+, WASH+, Numb+, ALDH1A1+, ALDH1+ |
|
Liver cancer |
CD13+,
CD133+, Lin28B+, SALL4+/ EpCAM+, CD90+/CD45−, CD44+/CD90+, SOX9,
β-catenin+/GEP |
|
Pancreatic cancer |
CD44+/CD24+/EpCAM+, CD133+/ CXCR4+, Pakt+/ SOX9+, CD133+/ CXCR4+,
FAM83A+, ALDH1A1+, CD133+/CD44+/CD24+/ESA+ |
Although
they tend to retain activation of one or more important and highly conserved
signaling pathways essential in the differentiation and pluripotency of stem
cell phenotypes, CSCs exhibit many characteristics of ESCs. CSCs, like ESCs,
which mature into blastocysts and supply nourishment for fetal growth, can
generate and sustain tumor growth. They can make tumor cells from a variety of
stem cells as well as normal somatic cells. They also have potential
transcription factors and surface indicators in common. They're also rich in
developmental signaling pathways that control embryonic cell characteristics,
proper organogenesis, and cell lineage differentiation, all of which could play
a role in the onset and advancement of poorly differentiated cancers. Tumor
cells have been found to contain five primary signaling pathways that confer
embryonic stemness (15).
The
Hedgehog, Hippo, Notch, TGF-, and Wnt/-catenin pathways were among them. All of
these routes are crucial for CSCs to be able to self-renew and transform into
similar daughter cells, preserving their immortality and allowing them to
differentiate into different types of cells. Furthermore, these pathways are
involved in the development, migration, and resistance of gastrointestinal
cancers. Because CSCs are so diverse, the expression of stemness pathways
fluctuates over time and in different types of gastrointestinal tumors.
Activation of CSC pathways has also been found in tumor cells that exhibit
specific CSC markers. In pancreatic cancer, for example, overexpression of
Notch1 and Notch2 has been related to higher expression of CD44 and EpCAM Wnt
signaling has been demonstrated to be active in CD44+ gastric CSCs to preserve
self-renewal and tumor growth. In CD133+ hepatocellular carcinoma (HCC) CSCs,
Notch and Jagged be strongly expressed (16).
Biomarkers
and signaling pathways specific to CSCs are important in differentiating
molecular categories with stem-like characteristics. Variable subtypes have
different levels of expression and activation of CSC biomarkers and signaling
pathways, which has led to research into potential new paths of therapeutic
strategies (Figure 1).

Figure 1. Treatments that target CSCs.
Anti-CSC medicines that target developmental pathways and CSC-associated
surface indicators have been identified.
Tumor plasticity
One of the key processes contributing to intratumor heterogeneity
has been suggested as cancer cell plasticity. In response to microenvironmental
stimuli, cancer cells can transition between a nontransformed differentiated
state and a tumorigenically transformed undifferentiated or CSC state.
Multilineage interconversion, dedifferentiation, and transdifferentiation are
all examples of stem cell plasticity (17). Normal stem
cells, progenitors, and/or differentiated somatic cells can all give rise to
CSCs. CSCs can develop into cancer cells, dedifferentiate back to their
original lineage cells, and/or transdifferentiate into other lineages (18, 19). Malignant
transformation is fueled by abnormally activated plasticity, which allows
tumors to adapt to the restrictions of tumor development and therapeutic
resistance. CHD1L was discovered to be a possible clinical developmental
lineage oncogene in HCC in a prior investigation. CHD1L expression is active
during embryonic development but gradually diminishes after terminal
differentiation. CHD1L expression, on the other hand, is abnormally elevated in
HCC. Elevated liver ancestral precursor markers and decreased hepatic lineage
differentiation markers accompany this dynamic expression pattern. Further
CHD1L inhibition may impede poorly differentiated HCC and make patients more
susceptible to chemotherapeutic treatments (20).
The link between CSCs and EMT has been discovered by providing
proof. But it is still debatable if EMT is required for CSCs, it is highly
significant in CSCs. Firstly, intermediate mesenchymal states are reversible at
earlier stages of development, depending on microenvironmental signals, and EMT
in tumor cells may be temporary, resulting in a more plastic CSC phenotype,
poorer patient survival, and more drug resistance. Six separate EpCAM-cell
populations characterized by the CSC markers CD61, CD106, and CD51, for
instance, displayed this intermediate EMT state and produced metastases more
efficiently (21). Furthermore, an increase in EMT
master transcription factors not only increases the metastatic potential and
increases tumor starting capacity (22). The EMT
phenotype is strongly associated with most gastrointestinal cancer subtypes
with stem cell characteristics.
In gastrointestinal cancers, molecular subgroups with CSC features
have been described.
Within tumors, gastrointestinal malignancies are exceedingly
variable, and molecular subtypes have been identified to help classify them.
Many cancers' transcriptomic, genomic, and/or epigenomic profiling provides the
foundation for molecular categorization. Different biological bases, such as
immunology, metabolism, and stemness, are reflected in these diverse molecular
subtypes. CSCs, in particular, are a key cause of intratumor heterogeneity.
Integrative molecular subclassification analyses from a CSC perspective may be
promoted to obtain a consensus molecular classification in patient prognosis
and therapy decisions.
Colorectal cancer CSC features categorization
Colorectal cancer stemness-based subtyping has received a lot of
attention, just like other gastrointestinal malignancies. C1 (21%) is
characterized by the suppression of pathways associated with EMT, C2 (19%) is
characterized by suppression of the Wnt pathway, C3 (13%) is characterized by
suppression of EMT, C4 (10) is described by increased expression of EMT and
genes linked to stem cell-like signatures, C5 (27%) is categorized by up-regulation
of Wnt pathway genes, and C6 (10) is characterized by upregulation of the EMT
pathway (23). The
transit-amplifying subtype is a heterogeneous subtype with a high concentration
of stem cell-relevant genes and the Wnt pathway, which may be separated into
two categories based on the differential cetuximab response (CS-TA and CR-TA).
Another stem-like subset is described by Wnt signaling target gene
overexpression and the presence of mesenchymal and myoepithelial stem-cell
characteristics, but also reduced expression of differentiation markers,
whereas the goblet-like and enterocyte subsets are enriched in
well-differentiated genes with few stem cell characteristics and low Wnt marker
expression (24). Using
meta-gene profiles to detect five primary subsets: surface crypt-like, lower
crypt-like, CIMP-H-like, mesenchymal, and mixed, in contrast to standard
molecular categorization based on gene expression profiling. Whenever the
mesenchymal subtype and mixed subtypes are enriched for high expression of the
EMT/stroma gene module, the surface crypt-like and lower crypt-like subtypes
are well distinguished with low expression of the EMT/stroma gene subsystem (25).
Gastric cancer CSC features categorization
The following four characteristics of the mesenchymal subtype are
CSC-like. For starters, this subtype is significantly linked to the activation
of the CSC pathway. Second, as compared to other varieties, it has high CD44
and low CD24 levels, which is similar to the QM-PDA subtype of PDAC. Third, it
maintains an undivided state, which is a crucial characteristic of CSCs.
Finally, genes expressed at low levels in HCC with hepatic stem cell features
have a considerable overlap with hypermethylated gene sets (26). Furthermore,
the proliferative subtype has increased activity for some carcinogenic
pathways: RAS, E2F, and MYC. MSI, MSS/EMT, MSS/p53+, and MSS/p53 are four
patient subtypes of gastric cancer, where MSS refers to microsatellite stable
tumors. The MSS/EMT module has a strong relationship with the EMT signature (27).
Esophageal cancer CSC features categorization
Esophageal cancer is divided into two categories based on
histology: esophageal adenocarcinomas (EACs) and esophageal squamous cell
carcinomas (ESCCs) (ESCCs). Molecular classification studies of esophageal
cancer are still scarce, in contrast to studies on other gastrointestinal tract
malignancies. ESCC1, ESCC2, and ESCC3 are the three ESCCs. SOX2 and TP63
amplification is common in ESCC1 malignancies. SOX2 is a pluripotent stem cell
transcription factor that promotes squamous epithelia formation and
maintenance. ZNF750 and NOTCH1 mutations, inactivation of the histone
demethylases KDM6A and KDM2D, deactivation of the PIK3CA inhibition PIK3R1 and
PTEN, and CDK6 amplification are all more common in ESCC2 tumors. The last
category, ESCC3, has mutations that predict RTK/RAS/PI3K pathway activation (28). A further investigation discovered
two unique ESCC subgroups. Subtype I contain a highly activated immune response
pathway, whereas subtype II contains pathways involved in ectoderm development.
Subtype II has an abundance of epithelial development genes such as E2F4, JUN,
KRT5, and KRT14. In Subtype II ESCC, PDPN and SIX1 have high expression levels,
and SIX1 can maintain or increase PDPN-positive CSCs. They uncovered potential
ESCC subset-specific diagnostic markers, including EYA2 and FOXA1 for subtype I
and KRT14 and LAMC2 for subtype II, that could aid in ESCC therapeutic practice
(29).
Treatments based on subtypes and
clinical relevance
Colorectal cancer subtypes
In De Sousa et al. study (30) evaluated the clinical
characteristics of CCS1 and CCS3 cancers and discovered that CCS1 tumors had an
excellent prognosis. At an early stage of adenomas, CCS3 tumors had malignant
potential and were resistant to anti-EGFR treatment The prognosis was better in
the surface crypt-like and lower crypt-like categories. CIMP-H-like and
mesenchymal subtypes were linked to poor overall survival (OS), with the former
additionally being linked to short relapse survival (SAR). There was a trend
toward the worse OS in the mixed subgroups (25). Type A has
the greatest prognosis, Type B has an intermediate prognosis but can benefit
from adjuvant 5-FU treatment, and Type C has the worst survival and resistance
to 5-FU-based chemotherapy, according to molecular categorization. Four
consensus molecular subtypes were discovered to be connected to clinical
characteristics while analyzing the existence of core subtype gene expression
patterns among current CRC subtyping techniques (31).
Gastric cancer subtypes
Gastric adenocarcinomas are divided into three types:
proliferative, metabolic, and mesenchymal. There were no significant variations
in survival between the three groupings, according to the analysis of survival
data. 5-FU therapy did not affect patients with tumors of the proliferative and
mesenchymal subtypes. PI3K-AKT-mTOR inhibitors were selectively responsive to
mesenchymal-subtype gastric cancer cells, probably because this subtype of
cells mimics CSCs. PI3K-AKT-mTOR inhibitors are also effective in prostate
cancer and glioblastoma, according to this report (32, 33). Another
distinguishing aspect of the mesenchymal subtype is the presence of high levels
of CD44. CD44 is a well-known CSC surface biomarker that is abnormally
expressed in a variety of malignancies as CD44s or CD44v (variant isoform).
CD44 overexpression is strongly linked to a malignant phenotype and poor
clinical outcomes. Sorafenib and 5-FU sensitivity was reduced in CD44-positive
cancer cells. Targeting CD44 for cancer treatment could be a promising method.
Antibodies to CD44 and inhibition of the HA-CD44 balance are two treatments
that can successfully decrease CSC characteristics in a variety of malignancies
(34).
Esophageal cancer subtypes
Esophageal cancer molecular categorization studies are still
restricted. Four subtypes are therapeutically significant. Subtype 1 was made
sensitive to the cell cycle checkpoint inhibitor CHFR. Furthermore, CDK4/6
inhibitors were beneficial across all subtypes, although CDK2 inhibitors were
more successful in patients with subtype 4 (35).
Conclusion
The most common genetic alterations and
tumor subtypes are gradually becoming more well-known, and their clinical
significance is becoming clearer. The biological characteristics and clinical
characteristics of gastrointestinal malignancies show substantial differences,
which are most likely due to heterogeneity. In clinical terms, heterogeneity is
mostly responsible for tumor development, metastasis, therapeutic resistance,
and relapse. The occurrence of molecular subtypes causes molecular
heterogeneity. CSC features are unquestionably linked to molecular categories
due to CSCs' substantial effect on heterogeneity. CSCs mimic embryonic stem
cells in appearance, implying the importance of developmental cues in cancer
onset and resistance to treatment. As a result, combining the molecular
subtypes linked to stemness features could reveal additional information about
treatment resistance. Other, more putative therapeutic, like scRNA-seq and
appropriate preclinical models, should be developed and employed in the precise
evaluation of intra- and intertumoral heterogeneity. More precise targeting of
tumor-initiating and driving events based on subtype-specific biomarkers could
be a unique therapeutic technique in the treatment of gastrointestinal cancer.
Eventually, comprehensive tumor and liquid biopsy procedures should be
developed to identify characteristic molecules that enable the whole molecular
profile to be defined and patient categorization to be determined. In
conclusion, we present an overview of molecular categorization from a CSC
perspective that may aid in the therapeutic management of patients with
gastrointestinal malignancies, resulting in better results.
Author contributions
SEN, FN, and SV wrote and compiled this article. AAS
wrote and edited the manuscript comprehensively. All authors confirmed the
final version of the paper.
Conflict of interest
The authors declare that they have no conflicts of interest.
References