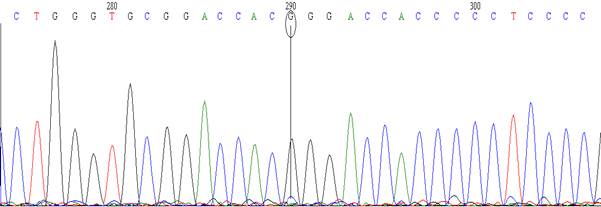
MT1A single
Nucleotide Polymorphism and Blood Mercury Levels
Maryam Salacheh 1, Amir Jalali
2*, Javad Babaei 1, Hamid Galehdari 3, Amal Saki 4
1 Department of Toxicology, School of Pharmacy and Toxicology
Research Center, Ahvaz Jundishapur University of
Medical Sciences, Ahvaz, Iran
2 Department of Applied Cellular Sciences and Tissue
Engineering, Langroud
School of Allied Medical Sciences and Medical Biotechnology Center, Guilan University
of Medical Sciences, Rasht, Iran
3 Department of Genetics, School of Sciences, Chamran
University, Ahvaz, Iran
4 Department of
Statistics, School of Health, Ahvaz Jundishapur
University of Medical Sciences, Ahvaz, Iran
*Corresponding
Author: Amir Jalali
* Email: amjalali@hotmail.com
Abstract
Introduction: The risk factors for the metallothionein (MT) polymorphism in
concentrations of heavy metals, especially mercury, in the blood are subject to
several confounding factors, including differences in the ethnicity of the
population analyzed, the sample size, and the type of the studied environment
heavy metals to which population is exposed. This study aimed to investigate
the effects of the MT1A(A>G) and MT1A(C>G) single nucleotide
polymorphisms (SNPs) on blood mercury levels in the city of Ahvaz (located in
southwest of Iran).
Materials
and Methods: 300 unexposed (control group) and 150 exposed (case group) were
included. DNA extraction, PCR-RFLP and DNA sequencing were performed, and blood
mercury levels were determined by AAS method with DMA-80.
Results: Blood mercury levels in the case group were higher than those in the
control group (p-value <0>G), with MT1A (C>G) polymorphism and
P-values of blood mercury levels of 0.69, 0.44, and 0.59. 0.56 for the case
and control groups, respectively. Results showed that these two SNPs were not
associated with mercury-induced toxicity in the case group despite high blood mercury
levels and exposure.
Conclusion: In conclusion, this takes look shows that MT1A (A>G) and MT1A
(C>G) polymorphisms aren't related to susceptibility to excessive blood
mercury attention in individuals.
Keywords: Mercury, Metallothionein, MT1A (A>G), MT1A(C>G), Iranian
population
Introduction
There
are three forms of mercury in the environment such as elemental, inorganic, and
organic mercury compounds (1). However, organic mercury (mostly methyl mercury)
exposure is via dietary fish consumption (2). Mercury is widely found in the
environment and foods and so are life-threatening organisms (1). Therefore,
mercury exposure may lead to toxicity. Genetic polymorphisms and individual
differences have a leading role in heavy metals toxic effects (3-4). The
different studies on the genetic susceptibility genes of heavy metals levels
have become of interest.
Metallothioneins
(MT) are cysteine-rich low molecular weight proteins. These proteins bind to
physiological and xenobiotic heavy metals (1). The
intracellular binding prevents the toxic effects and cellular damage of heavy
metals.
MT1, MT2, MT3 and MT4 are the main isoforms
expressed in humans. The Liver and kidney are the main prominent for synthesis
that needs dietary minerals (Zn, Cu, and Se) and amino acids (His and Cys) (2). Our study
focused on two SNPs of metallothionein in a regulatory region, including MT1A
missense (A>G) (rs8052394) and MT1A 5׳ near gene (C>G) (rs9922957).
There
are studies on MTs and their relation to heavy metal levels, such as cadmium,
lead, zinc and copper in blood samples (3, 4) and mercury levels in human urine and hair
samples (5). Also, there
is a relation to diseases such as the risk of ductal breast cancer (6), type2
diabetes mellitus (11), intestinal, and gastric cancer (12), lung cancer (13)
and inflammatory bowel disease (14). Furthermore, it was demonstrated that MT
gene polymorphism is related to metal levels in the placenta (15) and kidney
tissues (16).
MT
plays a crucial role in the detoxification of mercury blood concentration, and
altered gene coding was suggested as a potential role to explain high mercury
blood levels. The modification of neurobehavioral effects of mercury by genetic
polymorphisms of MT and the relationship between MT1A (A>G) and MT1A
(C>G) to hair and urine mercury level was studied (17). Individual
differences as a result of genetic polymorphisms lead to different adverse
effects of environmental factors (18). This genetic diversity can cause changes
in whole blood mercury levels. SNP in MTs may influence mercury biomarker
levels in the human body (19). Individual difference studies, for example, can
help doctors pinpoint an illness, suggest further tests and prescribe
appropriate drugs. Like MT genes, association studies of polymorphisms to heavy
blood levels should further investigate. This study attempted to investigate
the two MT polymorphisms as a dependent risk marker for mercury blood levels.
This is the first research on the relationship between SNPs of MT and heavy
metal levels in the Iranian population.
Materials and Methods
Study
population
This
study was performed following a protocol approved by the commission of
Bioethics at the Jundishapur University of Medical
Sciences, Ahvaz, Iran. This case-control study was performed in the Toxicology
Research Center (TRC). Whole blood samples that included 300 normal, healthy
volunteers (150 males and 150 females) with 25-70 years old (mean age
44.00±19.23) as the control group and 150 exposure people (75 males and 75
females) with 29-45 years old (mean age 37.30±8.19) as case group collected
from accredited medical diagnostic laboratories in Ahvaz city (southwest of
Iran) and factory workers exposed to mercury in Mahshahr
city (southwest of Iran), respectively, in the period from October 2014 to
December 2014. The control group was healthy blood donors having no evidence of
any personal or family history of high blood mercury concentration. Five
milliliters of venous blood were drawn into a sterile tube containing EDTA and
stored at -20 ºC until the isolation of genomic DNA. The subjects signed
informed written consent to take part in the study. Individuals filled out the
brief questionnaire. All the molecular analysis was performed in the Toxicology
Research Center of Jundishapur University of Medical
Sciences.
Genotype
analysis
Genotyping
was performed on DNA extracted from whole blood samples that were considered
for mercury assessments. Polymerase chain reaction based on the restriction
fragment length polymorphism (PCR-RFLP) was used for genotyping (Figures 1 and
2).
 Figure1. PCR-RFLP of MT1A missense (A>G) polymorphism on agarose gel 2% (paya
pajoohesh, Iran). In some cases, in addition to 743bp
bond, it's visible 405bp and 338bp bonds, which represent the heterozygous
genotype (AG).
Figure1. PCR-RFLP of MT1A missense (A>G) polymorphism on agarose gel 2% (paya
pajoohesh, Iran). In some cases, in addition to 743bp
bond, it's visible 405bp and 338bp bonds, which represent the heterozygous
genotype (AG).
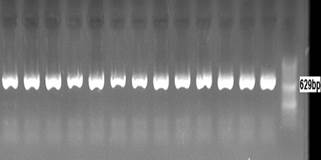
Figure2. Electrophoresis PCR products of
MT1A 5' near gene (C>G) polymorphism on agarose gel 1.5%.
Information
about the MT1A (A>G) and MT1A (C>G) and PCR conditions are listed in
Table 1.
Determination
of mercury levels
Whole
blood mercury was measured based on Atomic Absorption Spectrometry (AAS)
technique by Direct Mercury Analyzer (DMA-80, Italy) instrument. We entered
100µl of individual whole blood to DMA, and then the sample temperature rises
in the curve segment named Catalyst; the heated sample arrives at Amalgamator
segment that contains gold pieces for the release of mercury; eventually, the
atomized mercury absorbs light in Cuvette segment and determines the amount of
mercury according to light absorption and calibration curves (www.milestonesrl.com).
The
ethical approval of this study is IR.ajums.REC.1393.142. This code is addressed
at the following:
Behsan.ajums.ac.ir/webdocument/load.action/webdocument_code=1000&masterCode=33005332.
Statistical
analysis
Deviations from Hardy–Weinberg equilibrium was
tested using the Chi-square (χ2) test. Data analysis was performed by
Statistical Package for Social Sciences (SPSS) version 22 software. Values of P
< 0.05 were considered statistically significant.
Results
The
average whole blood mercury levels of exposure (the case group) and
non-exposure (control group) people were measured. It was found that the
amounts of mercury were 58.79±51 ppb and 6.65±3.5 ppb in the case and control
groups, respectively. The blood mercury levels in the case group are
approximately nine times higher than those in the control group. These results
show that there are significant differences (p value<0.001) between the two
groups of mercury; therefore, exposure to mercury in the case group has been
effective in increasing blood mercury levels.
Individuals
in the case and control groups were genotyped by DNA PCR-RFLP and DNA
sequencing techniques; genotype and allele frequencies were determined (20).
All genotype distribution did not diverge significantly from Hardy-Weinberg
equilibrium for both control and case groups separately. In the control group,
the genotype frequencies of MT1A (A>G) were 77%, 23% and 0.0% for wild-type,
heterozygous and homozygous; and allele frequencies were 88.5% and 11.5% for A
and G alleles, respectively. In case group for MT1A (A>G), the genotype and
allele frequencies were obtained 74% (AA), 26% (AG), 0.0% (GG) and 87% allele
A, 13% allele G. About MT1A (C>G) polymorphism the genotype and allele
frequencies were as follows: 92% wild-type, 8% heterozygous and 0.0%
homozygous, 96% allele C and 4% allele G in the control group and 80% wild-type,
20% heterozygous and 0.0% homozygous, 90% allele C and 10% allele G in case
groups. The genotype and allele frequencies P values were obtained by using the
Chi-Squire test. P values for MT1A (A>G) and MT1A (C>G) were 0.69 and
0.03, respectively.
Statistical
evaluation of gender (p=0.76) and age (p=0.60) significances in these two
polymorphisms was performed and no significant association was found.
In
the main part of the study, the results show that MT1A (A>G) and MT1A
(C>G) polymorphisms have no significant effects on mercury blood levels in the
case and control groups (Fig 4, 5). The results indicated that these two SNPs of
the MT gene were not associated with susceptibility to mercury blood
levels in the Ahvaz population of Southwest Iran. Mercury level was measured by
an AAS technique in male and female blood samples in exposure and non-exposure
groups and there was no significant difference between blood mercury levels in
female and male groups (p=0.73). The average blood mercury levels in people
with MT1A (A>G) and MT1A (C>G) polymorphisms that have wild-type,
heterozygous and homozygous genotypes demonstrate that SNP changes were not
considerable, however, about MT1A (C>G) SNP changes lead to an increase in
blood mercury level, however it is not significant (Table 2). A comparison of
blood mercury based on genotypes between case and control groups is shown on
the graph in figures 4 and 5.

Figure 4. The bar chart of the blood mercury concentrations of MT1A (A>G)
in case (n=150; p=0.69) and control (n=300; p=0.44) groups.
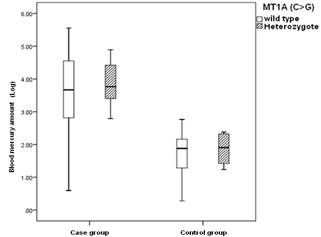
Figure 5. The bar chart of the blood mercury concentrations of MT1A (C>G)
in case (n=150; p=0.59) and control (n=300; p=0.56) groups.
Discussion
This report aimed to use a case-control study to establish a
database of the effect of MT1A (A>G) and MT1A (C>G) SNPs
in the Ahvaz population from Southwest Iran and to evaluate these SNPs as
an indicator of blood mercury level susceptibility.
There is an excellent mechanistic ground for finding an interaction
between the putative high-inducibility-associated MT genotypes and heavy metal
blood levels. For further comprehension of the role played by MT1A (A>G) and
MT1A (C>G) SNPs of MT gene in mercury blood levels, the two gene
polymorphisms were associated with mercury blood levels. In Iran country, the
industry is growing in particular the oil, and petrochemical industry and the
accumulation of toxic metals in the human body is increasing; thus, it is
necessary to use time and energy to investigate the effect and damage of toxic
metals in the body and identify the prevention methods (21). Expression of MTs
proteins increases via oxidative stress and heavy metal effect in the
regulatory area of the gene. MTs expression various in different tissues, so
there is a significant correlation between the metal level and MTs expression.
Induction of this protein is a highlight biomarker of heavy metal exposure
(22-24). MTs expressed polymorphically and these changes in polymorphism affect on proteins that bind to heavy metals for to
homeostasis and detoxification. MT1A (A>G) and MT1A (C>G) are two studied
MT polymorphism that is located in the regulatory region of chromosome 16 (25).
A mutation in one nucleotide, such as changing in nucleotide A and turning it
to nucleotide G in MT1A (A>G) and changing nucleotide C to nucleotide G in
MT1A (C>G) lead to SNP and may influence the toxicity of mercury. In this
study, the effect of these changes on blood mercury levels was investigated in
the Iranian population. There are several studies in the field of this
polymorphism such as polymorphism in MT1A (A>G) gene and the risk of type 2
diabetes mellitus in Chinese and Nepalese people. The results showed that the
incidence of type 2 diabetes was significantly related to G allele in SNP
rs8052394 (11, 26). Another study in the Italian central female population
proved that polymorphism in MT1A (A>G) gene coding region is associated with
longevity. Also, in Greece, it was found that the AG and GG genotype
significantly increased the risk of cardiovascular disease (27-28). It was
demonstrated that the variations of MT1A SNPs may influence urine uric acid and
N-acetyl-beta-D-glucosaminidase excretion in chronic
lead-exposed workers (29). The other reports were not found about the effects
of MT1A polymorphisms on mercury metabolism or toxicity. These reports are
unlike our results and SNP in MT1A (A>G) was effective. There is just one
study about MT1A (C>G) polymorphism in the USA population and there was no
significant relationship between MT1A (C>G) allele frequency and hair and
urine mercury levels in accordance with our results (9,19). These results may
display and explain that MT1A polymorphisms had no strong modifying effects on
mercury metabolism and toxicity. Much research has been done in the field of
metallothionein polymorphisms and heavy metal levels in the human body, which
the most typical are: A) The effect of metallothionein 2A-5A/G single
nucleotide polymorphism on blood Cd, Zn, Pb and Cu levels in the Turkish
population that highly statistically association were detected between MT2A and
these heavy metals except Cu (8). B) The association between 13 polymorphisms
of metallothionein and urine and hair mercury level were examined in the USA
population, and the results showed that there is no significant difference
between hair and urine mercury level and all of the polymorphisms except MT1M
(T>C) rs9936741. In this polymorphism individuals with TC genotype
significantly have high hair mercury levels than wild type genotype (19).
Our study is the first research about MT1A (A>G) rs8052394 and
MT1A (C>G) rs9922957 and their association with blood mercury levels. As
shown in Table 2, the mean amount of mercury blood levels in the MT1A (C>G)
SNP control group is more than MT1A (A>G) SNP control group. This study
identifies that MT1A (C>G) SNP changes may influence mercury blood
concentration and toxicity. Therefore, individuals with MT1A (C>G) SNP may
be more sensitive to mercury toxicity than MT1A (A>G) SNP (Figure 4,5). In
general, according to the results and other studies all over the world, it can
be stated that in most cases, the SNP is effective on heavy metals
concentration in the human body and it can say that should take more care of
because these people are susceptible to heavy metals. Thus, we can identify
more susceptible individuals. With the genetic database of people, safety
advice was earnest and they banned their exposure to heavy metals and other
toxins. It is hoped that in the future only by performing genetic testing of
people working in the industry and determining their genetic susceptibility to
heavy metals. There are several conceivable factors for the inconsistent
outcomes in the previous studies. First, the difference in the study design (sample
size, ethnicity, and selection of subjects) may have contributed. Second, it is
not easy to elucidate the relationship between genotype and phenotype. The
phenotype is often influenced by environmental factors in addition to genotype.
The perfect model would be to obtain cell lines that have MT1A (A>G) and
MT1A (C>G) SNPs of MT genes and their sensitivity towards mercury
studied.
Conclusions
In summary, our findings show that
MT1A (A>G) and MT1A (C>G) SNPs of MT gene don’t influence the Iranian
population's susceptibility to mercury blood levels. This result should be
confirmed in more studies on various ethnic groups
Author contribution
MS, JB, HG and AS collected the data and
compiled this article. AJ wrote and edited the manuscript
comprehensively. All authors confirmed final version.
Acknowledgments
This study was supported by the Deputy research of Jundishapur University of Medical Sciences. We acknowledge
Mr Miah for his aid to provide Blood samples. Also, the authors would like to
thank all participants who willingly contributed to the study.
Conflict of interest
The authors have declared that no competing interest exists.
Funding
This project was financially supported by the vice chancellor of
Research affairs of Ahvaz Jundishapur University of
Medical Sciences (Toxicology Research Center).
References
1.
Clarkson TW, Magos L. The toxicology of mercury and
its chemical compounds. Crit Rev Toxicol. 2006; 36(8): 609-662.
2. Clarkson, T. The three modern
faces of mercury Environ Health Perspect. Environ Health Perspect. 2002;110:11-23.
3. Gundacker C, Gencik M, Hengstschläger M. The relevance of the individual genetic background for the toxicokinetics of two significant neurodevelopmental
toxicants: mercury and lead. Mutat Res. 2010; 705(2): 130-140.
4. Guzzi G, La Porta, CA. Molecular mechanisms triggered by mercury.
Toxicology. 2008; 244(1): 1-12.
5. Sigel A, Sigel H, Sigel RKO. Metallothioneins and related
chelators: metal ions in life sciences.
Royal Society of Chemistry. 2009; pp.1-29
6. Binz, P
A, Kägi JH. Metallothionein: molecular evolution and
classification. Metallothionein IV, Springer. 1999; pp: 7-13.
7. Miura N. Individual susceptibility to cadmium toxicity and
metallothionein gene polymorphisms: with references to current status of
occupational cadmium exposure. Ind
Health. 2009; 47(5):487-94.
8. Kayaaltı Z, Aliyev V, Söylemezoğlu T. The potential
effect of metallothionein 2A− 5 A/G single nucleotide polymorphism on blood
cadmium, lead, zinc and copper levels. Toxicol Appl Pharmacol. 2011; 256(1):1-7.
9. Wang Y, Goodrich JM, Gillespie B, Werner R, Basu N, Franzblau A. An
investigation of modifying effects of metallothionein single-nucleotide
polymorphisms on the association between mercury exposure and biomarker levels.
Environ
Health Perspect. 2012; 120(4):530-4.
10 Krześlak A, Forma E, Jóźwiak P, Szymczyk A, Smolarz B, Romanowicz-Makowska H, Różański W, Bryś M. Metallothionein 2A genetic polymorphisms and risk of ductal
breast cancer. Clin Exp Med. 2014; 14(1):107-13.
11. Yang L, Li H, Yu T, Zhao H, Cherian MG, Cai L, Liu Y. Polymorphisms
in metallothionein-1 and-2 genes associated with the risk of type 2 diabetes
mellitus and its complications. Am. J. Physiol. Endocrinol. Metab. 2008; 294(5):E987-92.
12. Ebert MP, Günther T, Hoffmann J, Yu J, Miehlke S, Schulz HU, Roessner A, Korc M, Malfertheiner P. Expression of
metallothionein II in intestinal metaplasia, dysplasia, and gastric cancer.
Cancer Res. 2000; 60(7): 1995-2001.
13. Nakane H, Hirano M, Ito H, Hosono S, Oze I, Matsuda F, Tanaka H, Matsuo K.. Impact of
metallothionein gene polymorphisms on the risk of lung cancer in a Japanese
population. Mol Carcinog. Mol Carcinog. 2014; 54 Suppl 1:E122-8.
14. Brüwer
M, Schmid KW, Metz KA, Krieglstein CF, Senninger N, Schürmann G.
Increased expression of metallothionein in inflammatory bowel disease. Inflamm. Res. 2001;
50(6): 289-293.
15. Tekin D, Kayaaltı Z, Aliyev V, Söylemezoğlu T. The effects
of metallothionein 2A polymorphism on placental cadmium accumulation: is
metallothionein a modifiying factor in transfer of
micronutrients to the fetus? J. Appl. Toxicol. 2012; 32(4):270-5.
16. Kayaalti Z, Mergen G, Söylemezoğlu T. Effect of metallothionein core promoter region polymorphism on
cadmium, zinc and copper levels in autopsy kidney tissues from a Turkish
population. Toxicol Appl Pharmacol. 2010; 245(2):252-5.
17. Woods JS, Heyer NJ, Russo JE, Martin MD, Pillai PB, Farin FM. Modification of neurobehavioral effects of mercury by genetic
polymorphisms of metallothionein in children. Neurotoxicol Teratol. 2013;
39:36-44.
18. Olden K,
Wilson S.. Environmental health and genomics: visions and implications. Nature
Rev Gen. 2000; 1(2): 149-153.
19. Wang Y. A Gene-environment Study of Metallothionein Single
Nucleotide Polymorphisms, Mercury Biomarker Levels and Peripheral Nerve
Function, The University of Michigan.PhD thesis.
2011; pp:1-100
20. Babaei J, Jalali A,
Galehdari H, Saki, A. MT1A (A> G), MT1A (C> G),
MT1M (A> C) and MT4 (G> A) single nucleotide polymorphism allele
frequencies in Iranian populations. Biotech. Biotech. Equipment. 2016; 30(5):
1-7.
21. Farzin L, Amiri M, Shams H, Ahmadi Faghih M.A, Moassesi ME. Blood levels
of lead, cadmium, and mercury in residents of Tehran. Biol.
Trace Elem. Res. 2008; 123(1-3): 14-26.
22. Liu Y, Liu J, Habeebu SM, Waalkes MP, Klaassen CD.
Metallothionein-I/II null mice are sensitive to chronic oral cadmium-induced
nephrotoxicity. Toxicol Sci. 2000; 57(1):167-76.
23. Thirumoorthy N, Manisenthil Kumar KT, Shyam Sundar A,
Panayappan L, Chatterjee M. Metallothionein: an
overview. World J Gastroenterol. 2007; 13(7):993-6.
24.
Inoue K, Takano H, Shimada A, Satoh M. Metallothionein as an anti- Mediators Inflamm. 2009; 2009:101659.
25. Li Y, Maret W. Human
metallothionein metallomics. J Anal Atomic Spect. 2008; 23(8): 1055-1062.
26. Sharma SP, Dhakal B, Timilsina U. Evaluation
of the association of rs8052394 of metalothionein-1A gene with type 2 diabetes
mellitus in nepalese population.Indian
J. Res. Pharm. Biotech. 2013; 1(5): 570.
27. Cipriano
C, Malavolta
M, Costarelli
L, Giacconi
R, Muti E, Gasparini
N, Cardelli
M, Monti D, Mariani E, Mocchegiani
E. (2006). Polymorphisms in MT1a gene
coding region are associated with longevity in Italian Central female
population. Biogerontology. 2006; 7(5-6): 357-365.
28. Giacconi
R, Kanoni S, Mecocci P, Malavolta
M, Richter D, Pierpaoli
S, Costarelli
L, Cipriano
C, Muti E, Mangialasche
F, Piacenza
F, Tesei S, Galeazzi
R, Theodoraki
EV, Lattanzio
F, Dedoussis
G, Mocchegiani
E. Association of MT1A haplotype with
cardiovascular disease and antioxidant enzyme defense in elderly Greek
population: comparison with an Italian cohort. J. Nutr. Biochem. 2010; 21(10): 1008-1014.
29. Yang CC, Chen HI, Chiu YW, Tsai CH, Chuang HY. Metallothionein 1A polymorphisms may influence urine uric acid
and N-acetyl-beta-d-glucosaminidase (NAG) excretion
in chronic lead-exposed workers. Toxicology. 2013;
306:68-73.
 Figure1. PCR-RFLP of MT1A missense (A>G) polymorphism on agarose gel 2% (paya
pajoohesh, Iran). In some cases, in addition to 743bp
bond, it's visible 405bp and 338bp bonds, which represent the heterozygous
genotype (AG).
Figure1. PCR-RFLP of MT1A missense (A>G) polymorphism on agarose gel 2% (paya
pajoohesh, Iran). In some cases, in addition to 743bp
bond, it's visible 405bp and 338bp bonds, which represent the heterozygous
genotype (AG).