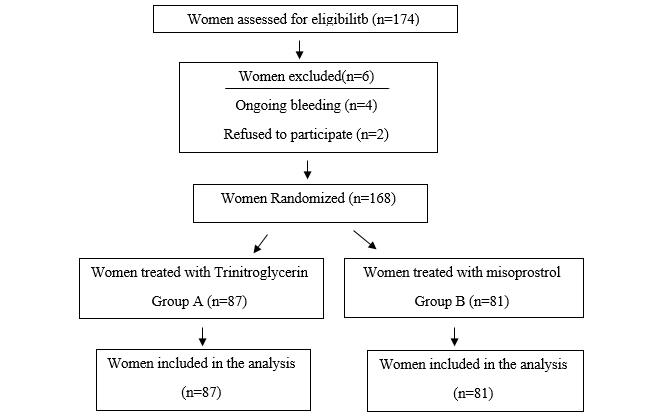
Figure 1.
Flow diagram of the clinical
trial.
Cervical ripening
before surgical evacuation of first-trimester pregnancy: a comparison between
misoprostol and trinitroglycerin
Fatemeh
Hosseinzadeh 1, Mandana Mansour Ghanaie 1*,
Roya Faraji 2, Ghazaleh Ghorbani 1, Seyedeh
Maryam Asgari Galebin 3, Sedigheh Pakseresht 4, Saman Marofizadeh
5, Seyed Mohammad Asgari Galebin 3
1 Reproductive Health Research Center,
Department of Obstetrics & Gynecology, Alzahra
Hospital, School of Medicine, Guilan University of
Medical Sciences, Rasht, Iran
2 Reproductive Health Research Center, Guilan University of Medical Sciences, Rasht, Iran
3 Guilan University of Medical Sciences, Rasht,
Iran
4 Department of Obstetrics, Community Health, Women Health Promotion,
Social Determinants of Health Research Center,
Reproductive Health Research Center, Shahid Beheshti
Nursing and Midwifery School, Guilan University of
Medical Sciences, Rasht, Iran
5 Department of Biostatistics, School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran
*Corresponding
Author: Mandana
Mansour Ghanaie
* Email: m_m_ghanaie@yahoo.com
Abstract
Introduction: Termination of pregnancy through curettage in the first trimester
requires cervical ripening (CR) which can be induced by medicinal or mechanical
methods. In the pharmaceutical method, vaginal administration of misoprostol as
well as vaginal trinitroglycerin (TNG) has been shown to induce effective CR.
This study was conducted with the aim of comparing vaginal misoprostol and
vaginal TNG in the CR of women candidates for the first-trimester curettage.
Materials
and Methods: This double-blind clinical trial study was conducted on 168 pregnant
women with a gestational age of less than 14 weeks who were candidates for
curettage. Participants were randomly divided into two groups receiving
vaginally either TNG (400 µgr) (n=87) or misoprostol (400 µgr) (n=81). Then,
the state of CR and the need for mechanical dilatation were compared between
the two groups. Also, the presence of any side effects caused by drug use was
determined.
Results: The percentage of CR in the misoprostol group (67.9%) was
significantly higher than the TNG group (32.2%) (P<0.001). Therefore, the
need for mechanical dilatation in the TNG group (66.7%) was significantly
higher compared with the misoprostol group (32.1%) (P<0.001). Also, the rate
of complications like diarrhea (9.9%) and abdominal pain (7.4%) in the
misoprostol group was significantly higher than in the TNG group (0%). However,
headache in the TNG group (34.5%) was significantly higher than the misoprostol
group (0%) (P>0.001). Generally, the rate of complications in the TNG group
(35.6%) was significantly higher compared with the misoprostol group (13.6%)
(P>0.001).
Conclusion: Vaginally Misoprostol is more effective than vaginally TNG on CR of
first-trimester curettage as well as it significantly reduces the need for
mechanical dilatation of the cervix.
Keywords: Misoprostol, Trinitroglycerin, Cervical ripening
Introduction
With
a prevalence of 15%, abortion is one of the most common complications of
pregnancy that occurs during the first and second trimesters. However,
regarding the excretion of pregnancy products due to an unfavorable cervix or
insufficient uterine contractions, evacuation of the residues with medicinal or
surgical methods is also possible (1).
According
to the definition of the World Health Organization (WHO), abortion is defined
as "termination of pregnancy before the 20th week of gestational age or a
fetus weighing less than 500 g (2). Induced abortion, as one of the
types of abortion, means the termination of pregnancy by medical or surgical
methods before the 20th week of pregnancy, which can be due to maternal or
fetal medical indications or spontaneous loss of the products of conception (3).In general, induced abortion is one
of the most common problems of pregnancy. More than 200,000 abortions are
performed in England every year, and about 90% of them occur in the first
trimester of pregnancy (4).
Some
maternal medical disorders such as cardiovascular diseases, hypertension,
diabetes, and malignancies, as well as fetal defects such as encephalitis,
trisomy, and myelomeningocele, lead to the termination of pregnancy (3). Today, medical termination of
pregnancy is more common due to surgical complications (5).
Although
curettage in the first trimester of pregnancy is associated with relatively few
complications, there is a possibility of damage to the cervix, uterine rupture,
and vaginal bleeding, especially in the adolescent age group and also in
primigravid women. In order to reduce these side effects, the cervix should be
softened to the desired extent. On the other hand, CR leads to a reduction in
the length of the operation and also greater patient satisfaction (6,7). Common methods used for CR include
both mechanical (for example, Foley catheters) and pharmacological (for
example, prostaglandins) methods (8–11).
Prostaglandins
are effective drugs for inducing abortion, which have different types and
include misoprostol (PGE1), tromethamine carboprost
(PGF2α) and dinoprostone (E2). Among these drugs,
misoprostol is more favorable because of its low risk, cheapness, and
availability compared to the other two drugs. By increasing the contractile
power of the uterus through direct stimulation of the myometrium, misoprostol
prepares the cervix and expels the remnants of pregnancy (12,13). According to the FIGO protocol, misoprostol with a medicinal dose
of 400 µgr is used for CR and for performing surgical curettage in the first
trimester of pregnancy (14,15). However, it has side effects such
as nausea, vomiting, diarrhea, and fever, which of course are reduced with
anti-nausea and anti-diarrhea drugs (16).
It
is sometimes difficult to tolerate these side effects, especially since CR
takes more than three hours (17). On the other hand, pregnant women
with a history of previous cesarean section and with the presence of uterine
scar, require more clinical care because of the possibility of uterine
perforation. In the study conducted by Ayati et al., misoprostol by both rectal
and vaginal methods with a dose of 800 µgr was effective for medical abortion
in the first trimester of pregnancy in women who had a history of cesarean
delivery. The remarkable thing in this study was that patients responded to the
treatment with different doses of medicine (18). Meanwhile, in the study of Roudsari et al., who examined the use of vaginal
misoprostol to terminate pregnancy in the first trimester, more than one-third
of the participants had positive results with the second dose (19). Currently, in addition to
prostaglandins, including misoprostol, nitrite oxide (NO) compounds are also
recommended for CR before curettage (8,20,21). The advantage of using TNG
compared to misoprostol is CR without causing contraction in the uterus, also
it can be easily and safely used on an outpatient basis (8,20). But prostaglandins still remain the best compounds in this regard;
for their fewer side effects. They are easier to accept and seemingly safer
than prostaglandins like misoprostol in cases such as women with a history of
cesarean section or uterine scars (8,20). The results of a study by
Sharifzadeh et al. showed that there was no statistically significant
difference in CR after using misoprostol with a dose of 400 µgr and TNG with a
dose of 1200 µgr, therefore, they introduced TNG as a suitable substitute for
misoprostol in CR (22).
The present study was conducted with the aim of comparing misoprostol and TNG
in the CR of women candidates for curettage because limited research has been
done on effective drugs for CR in women candidates for first trimester
curettage. Also, few studies have compared the effect of TNG and misoprostol
and other related issues like the contradictory results obtained regarding the
comparison of the two drugs as well as their effective dose for curettage; and
how to reduce the drug complications in pregnant women.
Materials and Methods
The
study protocol was a double-blind randomized study conducted according to the recommendations in the CONSORT statement (23). The study was approved by the
research ethics committee of Guilan university of
science with an ethical code of IR.GUMS.REC.1400.044 and an IRCT clinical trial
code of IRCT20210510051247N1. One
hundred sixty-eight women with gestation age of less than 14 weeks and Bishop
score ≤ 4, scheduled for surgical termination of pregnancy by suction
curettage, agree to participate in this study from June 2021 to September 2022.
Inclusion
criteria were healthy women candidates
for curettage with a gestational age of less than 14 weeks, body temperature of
less than 38 0c, a normal heart rate ranging 70-100 bmp and a
systolic blood pressure between 100-130 mm Hg, no narcotic consumption, no
coagulation disorder, no chronic diseases (such as active liver disease,
cardiovascular disease, uncontrolled seizures, history of glaucoma, suffering
from adrenal disease), no previous cervical surgery, and no more than one
uterine scar. Exclusion criteria included the patient's unwillingness to
continue participating in the study as well as ongoing bleeding, and allergy to
either IMN or misoprostlol.
The
sample size was calculated using G*Power statistical software, version 3.1.
Considering that the main purpose of the plan was to compare the ratio of CR
between the two groups of TNG and misoprostol, the sample size method was used
to compare the two ratios. To determine the sample size, a type I error of 0.05
and a type II error of 0.2 (power 0.8) were set. Also, according to the study
of Dabiri et al. (24), the ratio of CR in the two groups
was considered to be 0.5 and 0.7 respectively. According to the above
mentioned, the sample size was equal to 74 people in each group (total sample
size: 148 people), but because of the possible loss of samples (15%) and in
order to increase the accuracy of the study, 88 people were placed in each
group, eventually.
After
obtaining a written consent form eligible women in the study, demographic
information of the participants was recorded through a checklist which included
age, gravidity, parity, gestational age, height, weight, body mass index (BMI),
education level, employment status, history of cesarean, hypertension,
diabetes, blood disorders or other diseases, drug sensitivity, and the cause of
curettage.
Primary cervical examination was performed by
a resident of gynecologist, and if the cervix was closed, the case was selected
randomly using the Sealed Envelope Ltd. 2019 and placed with online
randomization service in one of the two groups (A, B). The participants in group A were given 400
µgr of TNG vaginally and in group B they received 400 µgr of misoprostol, which
is equivalent to two 200 µgr of misoprostol, as the same way.
Since
the drugs were given to the patient by list, by another resident of gynecology,
neither parcipating women
nor the
surgeon who performed the surgical abortions ,
were aware of wheater
IMN or misoprostol had been
administered. In case of no response to
the drug, 4 hours after drug taking, the patients in both groups were examined
again by another resident of gynecology, completely unaware of the condition of
the groups. If the cervix was completely closed and consistently firm, the
result was considered negative, so the patient was not transferred to the
operating room for the bougie dilatation. If the cervix was relatively soft or
had dilatation, the patient was transferred to the operating room for curettage
and was examined with a bougie dilator Under general anesthesia. The bougie is
a device that is used to mechanically dilate and prepare the cervix in the
operating room. This device is available in different sizes with a diameter of
1-26 mm and numbers 1-8, which is used to open the cervix. Passing a bougie
with a size equal or up to 8 mm through the cervix indicates a positive
successful response to the drug, therefore the patient can undergo curettage
without the need for mechanical dilatation. If an 8 mm Hegar bougie didn’t
pass, it was considered a negative and unsuccessful failure treatment method.
Curettage was performed after gentle mechanical dilatation with a Hegar bougie.
The
questionnaire concerned symptoms such as: abdominal pain, diarrhea, hypotension
(BP ≤ 90 mmHg), headache, vaginal bleeding and palptations.
Statistical
analysis
The
values of quantitative variables are shown as "standard deviation ±
mean" and the values of qualitative variables are shown as "%
frequency". In order to compare individual and clinical variables between
the two groups of TNG and misoprostol, the independent t-test was used for
quantitative variables and the chi-square test (or Fisher's exact test) was
used for qualitative variables. For statistical comparison of CR between the
two groups, the chi-square test was used and for statistical comparison of the
frequency of side effects, fishers exact test and chi-square test were used.
The
data was analyzed using SPSS version 16 software and a significance level of
0.05 was set. P–value <0.05 was considered statically significant.
Results
Of
the 174 women recruited to the study; 2 women decided to discontinue their
participation in the study and 4 people were omitted due to excessive bleeding.
A
total of 40 people from the TNG group and 62 people from the misoprostol group
were transferred to the operating room for curettage.
28
people from the TNG group and 55 people from the misoprostol group had positive
results, it implies that the Hegar bougie no.8 was removed gently without any
pressure, through the cervix.
The
personal, social, and clinical characteristics of the 168 participants are
shown in Table 1. The average age of women was 30.76 ± 6.59 years. The average
gestational age of women was 38.15 ± 2.16 weeks. Of the 168 cases studied, 66
(39.3%) had a university education and 58 (34.5%) were employed. The pregnancy
of 59 people (35.1%) was G1, 57 people (33.9%) were G2, and 52 people (31.0%)
were G3 or more. 63 cases (37.5%) had no children, 77 cases (45.8%) had one
child, and 28 cases (16.7%) had two or more children. 7 cases (4.2%) had
hypertension, 14 cases (8.3%) had diabetes, 37 cases (22.0%) had other
diseases, and 4 cases (2.5%) had drug sensitivity. The reason for curettage was
residual pregnancy in 110 people (65.5%), 50 people (29.8%) had other reasons
for abortion, including missed-abortion, blighted ovum as well as lack of fetal
heart formation, and 8 people (4.8%) were under forensic medicine
justification.
There
was no statistically significant difference between the two groups of TNG and
misoprostol in terms of all individual, social and clinical variables
(P>0.005); In other words, the women candidates for curettage in the two
investigated drug groups were homogenous (similar) in terms of all individual,
social and clinical variables (Table 1).
Table 1. Baseline characteristics of study
population.
|
|
Miso (n= 81) |
TNG (n=87) |
Total (n= 168) |
T/x2 |
P- value* |
|
|
Maternal,
y, mean (±SD) |
31.44±6.47 |
30.11±6.67 |
30.76±6.59 |
1.31 |
0.192† |
|
|
height,
cm, mean (±SD) |
158.70±4.38 |
158.26±4.05 |
158.48±4.21 |
0.67 |
0.500 † |
|
|
Weight,kg, mean (±SD) |
68.56±11.80 |
70.01±12.27 |
69.31±12.03 |
0.78 |
0.435† |
|
|
BMI,
kg/m2, mean (±SD) |
27.28±4.93 |
27.97±4.87 |
27.97±4.84 |
0.91 |
0.361 † |
|
|
GA,
wk, mean (±SD) |
8.03±2.40 |
8.24±1.97 |
8.15±2.16 |
0.36 |
0.563
† |
|
|
Gravidity,
mean (±SD) |
2.00±0.96 |
2.21±1.29 |
2.11±1.14 |
1.19 |
0.237 † |
|
|
Parity,
mean (±SD) |
0.79±0.74 |
0.86±0.84 |
0.83±0.79 |
0.59 |
0.556
† |
|
|
Cause
of curettage |
ROP |
54 (66.7) |
56 (64.4) |
110 (65.5) |
0.10 |
0.754 †† |
|
others |
27
(33.3) |
31
(35.6) |
58
(34.5) |
|||
|
Education,
n (%) |
Academic |
27 (33.3) |
39 (44.8) |
66 (39.3) |
2.60 |
0.107 †† |
|
N-Academic |
54
(66.7) |
48
(55.2) |
102
(60.7) |
|||
|
Employment |
employed |
58 (34.5) |
35 (40.2) |
23 (28.4) |
1.61 |
0.447 †† |
|
housekeeper |
58
(71.6) |
52
(59.8) |
110
(65.5) |
|||
|
c/s
history, n (%) |
26 (32.1) |
26 (29.9) |
52 (31.0) |
0.08 |
0.756 †† |
|
|
HTN,
n (%) |
4
(4.9) |
3
(3.4) |
7
(4.2) |
- |
0.712
** |
|
|
Diabet,
n (%) |
6 (7.4) |
8 (9.2) |
14 (8.3) |
0.18 |
0.675 †† |
|
|
Other
diseases, n (%) |
20
(24.7) |
17
(19.5) |
37
(22.0) |
0.65 |
0.421
†† |
|
|
Drug
allergy, n (%) |
3 (3.8) |
1 (1.2) |
4 (2.5) |
- |
0.350 ** |
|
Data are mean (SD) or n(%)
Miso, misoprostol; TNG, trinitroglycerin; BMI,body mass index; C/S, cesarean section; HTN,
hypertension; ROP, retain product of conception.
*p> 0.05 significant statistical
difference between groups (student t test or x2 test)
† Independent t test
†† x2 test
** Exact fisher test
So,
the CR rate in the misoprostol group (67.9%) was significantly higher than the
TNG group (32.2%) but this difference was not statistically significant
(P<0.001) (Table 2).
Table 2. cervical ripening, mechanical dilatation &
causes of curettage.
|
|
Miso (n=
81) |
TNG
(n=87) |
x2 |
P-value* |
||
|
Result |
CR |
Yes |
55 (67.9) |
28 (32.2) |
21.41 |
<0.001 †† |
|
No |
26 (32.1) |
59 (67.8) |
||||
|
MD |
Yes |
26(32.1) |
58(66.7) |
20.05 |
<0.001 †† |
|
|
No |
55(67.9) |
29(33.3) |
||||
|
Cause |
ROP |
Yes |
40 (74.1) |
15 (26.8) |
24.59 |
<0.001 †† |
|
No |
14 (25.9) |
41 (73.2) |
||||
|
other |
Yes |
15 (55.6) |
13 (41.9) |
1.07 |
0.300 †† |
|
|
No |
12 (44.4) |
18 (58.1) |
||||
Data are n (%)
CR, Cervical Ripening; MD,
Mechanical Dilatation; Miso, Misoprostal; TNG,
Trinitroglycerin
ROP, Residue of pregnancy
*P<0.05 Significant Statistical difference between
groups
†† x2 test
Diarrhea
was a common side effect in women treated with misoprostol. The rate of
diarrhea in the misoprostol group (9.9%) was significantly higher than the TNG
group (0%) (P=0.002); also, the rate of abdominal pain in the misoprostol group
(7.4%) was significantly higher than the TNG group (0%) (P=0.011). The most
common side effect after TNG was headache. The rate of headache in the TNG
group (34.5%) was significantly higher than the misoprostol group (0%)
(P<0.001). According to the findings, there was no statistically significant
difference between women receiving TNG and misoprostol in terms of palpitations
(P=0.683) and hypotension (P=0.498). Generally, the rate of complications in
the TNG group (35.6%) was significantly higher than the misoprostol group
(13.6%) (P<0.001) (Table3).
Table 3. Side effects in groups.
|
Complication |
Miso
(n=81) |
TNG (n=82) |
x2 |
P-Value |
|
Diarrhea |
8 (9.9) |
- |
- |
0.002 † |
|
Abdominal Pain |
6 (7.4) |
- |
- |
0.011 † |
|
Palpitations |
2 (2.5) |
4 (4.6) |
- |
0.683 † |
|
Hypotension |
- |
2 (2.3) |
- |
0.498 † |
|
Headache |
- |
30 (34.5) |
34.0 |
<0.001
†† |
|
General |
11 (13.6) |
31 (35.6) |
10.88 |
>0.001†† |
Data are n (%)
** Exact fisher test
††: x2 test
In women whose curettage was due to retained products of
conception, the rate of diarrhea in the misoprostol group (13.0%) was
significantly higher than the TNG group (0%) (P=0.006); also, the rate of
abdominal pain in the misoprostol group (9.3%) was significantly higher than
the TNG group (0%) (P=0.026), while the rate of headache in the TNG group
(32.1%) was significantly higher compared with the misoprostol group (0%)
(P<0.001). According to the findings, there was no statistically significant
difference between women receiving TNG and misoprostol in terms of palpitations
(P=0.615) and hypotention (P=1.000). Generally, the
rate of complications in the TNG group (32.1%) was higher than the misoprostol
group (16.7%), but this difference was not statistically significant (P=0.059).
In
women whose curettage was caused by other reasons, the rate of headache in the
TNG group (38.7%) was significantly higher than the misoprostol group (0%)
(P<0.001).
According
to our findings, there is a statistically significant difference between women
receiving TNG and misoprostol in terms of diarrhea (P=0.466), abdominal pain
(P=0.466), palpitations (P=0.240) and hypotention
(p=0.0001). Generally, the rate of complications in the TNG group (41.9%) was
significantly higher than in the misoprostol group (7.4%) (P=0.003) (Figure1).

Figure 1.
Flow diagram of the clinical
trial.
Discussion
In this clinical trial, the therapeutic effect of TNG and
misoprostol on CR was compared in women candidates for curettage in Rasht,
Iran. The results of our research showed a significant difference regarding the
effectiveness of TNG and misoprostol for CR among women candidates for
curettage. Therefore, the need for the
procedure reduces the need for mechanical dilatation. As the research
continued, frequency of side effects caused by the use of drugs was compared
between the two groups. Our results showed that diarrhea and abdominal pain
were the most common side effects of misoprostol with 9.9% and 7.4%
respectively, while general side effects and headache were 35.6% and 35.6%
respectively. Headache with a rate of 34.5% was the most common side effect
caused by the use of TNG. However, TNG did not have severe side effects for
patients. Several studies compared the effectiveness of these two drugs on CR
of women candidates for curettage. In a double-blind randomized clinical trial
by Sharifzadeh et al., the effect of TNG and misoprostol on CR for curettage
was compared in 60 pregnant women in their first trimester. The results of
their research showed that the level of effectiveness of misoprostol and TNG on
CR in the two groups of study had no statistically significant difference. These
researchers concluded that TNG can be used as a suitable substitute for
misoprostol in CR. The results of our research are inconsistent with the
findings of this research. In our research, which had a larger sample size,
misoprostol was not only more effective in CR, but also led to a significant
reduction in the need for mechanical dilatation compared to TNG (22).
In another study, Zhuo et al., compared the efficacy and safety of
vaginal and oral administration of misoprostol in CR compared with the placebo
group. The results of their research showed that the width of the cervix in
women receiving vaginal and oral misoprostol was 7.2 and 7.5 mm, respectively,
which was significantly more compared with the placebo group. The time needed
for CR in both groups receiving vaginal (75 seconds) and oral (82 seconds)
misoprostol was significantly less than the placebo group (148 seconds) (25).
In a study, Dabaghi et al., investigated
the effect of vaginal misoprostol on CR in 60 pregnant women candidates for
D&C compared with the placebo group. The results of their research showed
that the vaginal misoprostol can be a suitable drug for CR before performing D&C,
and it also led to an easier dilatation of the cervix (26). The results of our research are
somewhat comparable with their study. However, in our study, TNG was used
instead of the placebo group, and the results presented showed that misoprostol
is a better option compared to TNG in CR.
In another clinical trial research, Francis et al. compared the
effectiveness of two doses of 25 and 50 µgr of misoprostol on CR at the
termination of pregnancy. The results of their research did not report any
statistically significant difference in the effectiveness and safety of each
dosage regimen compared to the other one (27).
Also, Radulovic et al. in a study compared the effectiveness of
misoprostol and isosorbide mononitrate (IMN) before curettage in the first
trimester of pregnancy on CR of 120 women with a gestational age of fewer than
14 weeks, who were candidates for curettage (20).
The results of this research showed that misoprostol is more
effective than IMN in preparing and CR, but it had more side effects such as
abdominal pain, nausea, and bleeding, while the most common side effect of IMN
was headache.
In our research, misoprostol had much better effectiveness compared
to TNG. On the other hand, the most common side effects of misoprostol were
diarrhea and abdominal pain.
In another clinical trial study, Teimouri et al. compared the
effect of two drugs; vaginal misoprostol and vaginal TNG on CR in 148
primigravida patients with full-term pregnancies. The results of their research
showed that compared to TNG, misoprostol causes faster and more effective CR
and is associated with fewer side effects(28). Our results are completely
consistent with the findings of their research. Therefore, according to the
results of this research and previous studies, vaginal misoprostol had a
stronger effect on CR of women who were candidates for curettage compared to
vaginal nitroglycerin.
Conclusions
The results of this study showed
that vaginal misoprostol has stronger effect on CR in participants and reduced
the need for mechanical dilatation, compared to vaginal TNG with the usual
dosage.
Although vaginal TNG with a dose of
400 µgr, is less effective than vaginal misoprosterol.
It does not have any dangerous side effects, and it can be a substitute for
misoprostol, if misoprostol is not available or in case of limitation, such as
patient's sensitivity.
Author contribution
FH, MMGh. Conceptualization:
RF. Data collection: GhGh, SMAG.
Formal Analysis: SM. Writing, review and editing: SMAG. Writing,
original draft: MMGh, GhG.
Conflict of interest
The authors report no conflict of interest regarding the
publication of this paper.
Funding
No funding.
Acknowledgments
This article has been taken from the dissertation of Ms. Ghazaleh Ghorbani.
References
1. Tesfaye B, Tewabe M, Ferede A, Dawson A.
Induced second trimester abortion and associated factors at debre Markos
referral hospital: cross-sectional study. Women’s Heal.
2020;16:1745506520929546.
2. Cunningham FG, Leveno
KJ, Bloom SL, Spong CY, Dashe JS, Hoffman BL, et al. Williams obstetrics. Vol.
7. McGraw-Hill Medical New York; 2022.
3. De Londras F, Cleeve A,
Rodriguez MI, Lavelanet AF. The impact of ‘grounds’ on abortion-related
outcomes: a synthesis of legal and health evidence. BMC Public Health.
2022;22(1):1–14.
4. Sam S, Tai-MacArthur S,
Shangaris P, Sankaran S. Trends of Selective Fetal Reduction and Selective
Termination in Multiple Pregnancy, in England and Wales: a Cross-Sectional
Study. Reprod Sci. 2021;1–8.
5. Van Look PFA,
Cottingham J. The World Health Organization’s safe abortion guidance document.
Am J Public Health. 2013;103(4):593–6.
6. Sekiguchi A, Ikeda T,
Okamura K, Nakai A. Safety of induced abortions at less than 12 weeks of
pregnancy in Japan. Int J Gynecol Obstet. 2015;129(1):54–7.
7. Allen RH, Goldberg AB.
Cervical dilation before first-trimester surgical abortion (< 14 weeks’
gestation). Contraception. 2016;93(4):277–91.
8. Mansour Ghanaie M,
Mirblouk F, Godarzi R, Shakiba M. Effect of outpatient isorbide mononitrate on
success of labor induction. J Babol Univ Med Sci. 2013;15(2):12–7.
9. Mandana Mansour G, Mina
J, Forozan M, Seyed Alaedin A, Morteza Fallah K. < A> randomized
controlled trial of foley catheter, extra-amniotic saline infusion and
prostaglandin E2 suppository for labor induction. 2013;
10. Ghanaie MM, Jafarabadi
M, Milani F, Asgary SA, Karkan MF. A randomized controlled trial of foley
catheter, extra-amniotic saline infusion and prostaglandin e2 suppository for
labor induction. J Fam Reprod Heal. 2013;7(2):49.
11. Ghanaei MM, Asgharnia M,
Farokhfar M, Ghalebin SMA, Rafiei E, Haryalchi K. The effect of consuming
evening primrose oil on cervical preparation before hysteroscopy: An RCT. Int J
Reprod Biomed. 2022;591–600.
12. Ohannessian A,
Baumstarck K, Maruani J, Cohen-Solal E, Auquier P, Agostini A. Mifepristone and
misoprostol for cervical ripening in surgical abortion between 12 and 14 weeks
of gestation: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol.
2016;201:151–5.
13. Kulier R, Kapp N,
Gülmezoglu AM, Hofmeyr GJ, Cheng L, Campana A. Medical methods for first
trimester abortion. Cochrane database Syst Rev. 2004;(1).
14. Wu H, Marwah S, Wang P,
Wang Q, Chen X. Misoprostol for medical treatment of missed abortion: a
systematic review and network meta-analysis. Sci Rep. 2017;7(1):1–9.
15. Raymond EG, Harrison MS,
Weaver MA. Efficacy of misoprostol alone for first-trimester medical abortion:
a systematic review. Obstet Gynecol. 2019;133(1):137.
16. Sharami S, Milani F,
Hosseini F, Fakoor F. Pre-operative Rectal Misoprostol before Myomectomy affect
Bleeding in Patients with Intramural Myoma. Pakistan J Med Heal Sci.
2020;14(2):888–92.
17. Strelow M, Maissiat J,
Savaris MS, da Silva DM, Savaris RF. Lower and extended dosage of misoprostol
for cervical ripening in 1st trimester miscarriage (MISO200): A randomized
clinical trial. Eur J Obstet Gynecol Reprod Biol. 2022;269:30–4.
18. Ayati S, Vahid Roudsari
F, Banavi M, Shakeri MT, Berahmat A. Comparison between rectal misoprostol and
vaginal misoprostol for first trimester termination of pregnancy in patients
with previous uterus surgery. Iran J Obstet Gynecol Infertil. 2013;15(42):1–6.
19. Roudsari FV, Ayati S,
Ghasemi M, Mofrad MH, Shakeri MT, Farshidi F, et al. Comparison of vaginal
misoprostol with foley catheter for cervical ripening and induction of labor.
Iran J Pharm Res IJPR. 2011;10(1):149.
20. Radulovic N, Norström A,
Ekerhovd E. Outpatient cervical ripening before first‐trimester surgical
abortion: a comparison between misoprostol and isosorbide mononitrate. Acta
Obstet Gynecol Scand. 2007;86(3):344–8.
21. Kim SF. The role of
nitric oxide in prostaglandin biology; update. Nitric Oxide. 2011;25(3):255–64.
22. Sharifzadeh FS, Dezfooli
GA, Chagharvand MM, Azad Z. Comparison of the efficacy of Glyceryl trinitrate
and Misoprostol on cervical ripening for curretage in pregnant women at first
trimester. Iran J Obstet Gynecol Infertil. 2015;18(174):6–11.
23. Cui Q, Tian J, Song X,
Yang K. Does the CONSORT checklist for abstracts improve the quality of reports
of randomized controlled trials on clinical pathways? J Eval Clin Pract.
2014;20(6):827–33.
24. Dabiri Oskei A, Bayat F,
Moghimi Haji Z, Kolifarhood G. Individual and combined administration of
intravaginal misoprostol and transcervical foley catheter in cervical ripening
in nulliparous women. Iran J Obstet Gynecol Infertil. 2018;21(2):16–23.
25. Zhuo Z, Yu H, Gao L,
Jiang X. Effectiveness of misoprostol administration for cervical ripening in
women before operative hysteroscopy: a randomized, double-blinded controlled
trial. Minim Invasive Ther Allied Technol. 2019;28(6):344–50.
26. Talat D, Khadijeh E,
Sara G. Evaluation of the effect of vaginal misoprostol on cervical priming in
patients candidate for dilatation and diagnostic curettage: a randomized
clinical trial. 2013;
27. Adebayo FO, Onafowokan
O, Adewole N. A Comparison of 25 µg with 50 µg Misoprostol for Cervical
Ripening and Induction of Labor. J Women’s Heal Care. 2017;6(378):420–2167.
28. Teimouri B, Ghasemi M,
Sakhavar N, Khajeh Noori S. Comparison of vaginal trinitroglycerin (TNG) and
vaginal misoprostol in cervical ripening at term pregnancy. Iran J Obstet
Gynecol Infertil. 2018;20(11):8–14.