Anti-Mullerian
hormone level in relation to physical activity and reproductive determinants in
North Iranian infertile women
Roya Kabodmehri 1, Seyedeh Hajar Sharami 1*,
Forozan Milani 1, Nasrin Ghanami Gashti 1, Mahboubeh
Eftekhari 1, Ali Aghazadeh 1
1 Reproductive Health Research Center, Department of Obstetrics
& Gynecology, Al-zahra Hospital, School of Medicine, Guilan University of
Medical Sciences, Rasht, Iran
Corresponding Authors: Seyedeh
Hajar Sharami
* Email: sharami@gums.ac.ir
Abstract
Introduction: Female infertility is responsible for approximately half of all cases
of infertility and one of the causes of infertility in women is related to
ovarian disorders. Anti-Müllerian
Hormone (AMH) is one of the clinical markers of ovarian reserve. Physical
activity may affect the reproductive system and AMH concentration in serum.
We aim to evaluate the relationship between physical activity and
reproductive determining fertility and anti-mullerin hormone (AMH) in infertile
women in northern Iran.
Materials and Methods: This cross-sectional
study included 234 women aged 18–45 referred to the Infertility Clinic of the
Al-Zahra Hospital, Rasht, Iran. The reproductive characteristics and the amount
of physical activity of the patients were recorded. Exclusion criteria included
menopause, cancer, underlying endocrine diseases, use of hormonal drugs,
diagnosis of PCOS based on Rotterdam criteria, any ovarian and uterine surgery,
and endometriosis.
Results: As expected, we observed significantly lower AMH concentrations in
older participants. There was no association between reproductive determinants
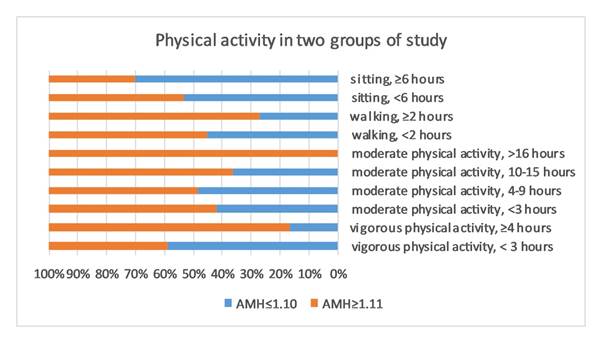
and AMH level (P> 0.05). We observed lack of physical activity as well as
vigorous physical activity, is associated with lower AMH concentration (P=
0.025, and P= 0.039 respectively).
Conclusion: In this study, AMH levels appear to be significantly lower in patients
with a lack of physical activity as well as vigorous physical activity. The
results of this study showed that by improving lifestyle, including the
appropriate amount of physical activity, it may be possible to improve the
results of infertility treatments. However, a larger study is needed to verify
the findings of this study.
Keywords: Anti-Mullerian hormone, AMH, Female infertility, Physical activity, Lifestyle
Introduction
Female
infertility is responsible for approximately half of all cases of infertility
and one of the causes of infertility in women is related to
ovarian disorders. anti-mullerin hormone (AMH) is produced by the granulosa cells of
pre-antral and small antral ovarian follicles and is widely accepted as a
clinical marker of ovarian reserve (1). It is a member of the transforming
growth factor-β superfamily, and there is a strong positive correlation between
circulating AMH concentrations and the number of follicles in the ovary (2). Since the number of follicles is
well correlated with the level of AMH (3), it can reflect the number of
dormant follicles in adult women. AMH suppresses the cyclic recruitment
of primordial follicles into the pool of growing follicles and its levels
decrease with age (4,5), thus serving as a marker of female
reproductive aging (6). AMH level is highly variable among
women, even measured on the same day in the menstrual cycle. Serum AMH level
has been reported to be a highly accurate tool for the diagnosis of polycystic
ovary syndrome (PCOS) (7) and premature ovarian insufficiency
(8). In addition, AMH is used for the
prediction of ovarian response during in vitro fertilization (IVF) treatment,
and prediction of age at menopause (9,10).
The
fact that AMH can't predict the probability of a woman conceiving within a
given period may be related partly to variation of circulating AMH even within
the same age in different women (11) due to various lifestyle and
reproductive characteristics. Konishi et al. (2014) examined the association
between AMH levels and menstrual cycle and lifestyle characteristics among
young Japanese women. They reported that circulating AMH concentration was
significantly lower among young women who had more severe menstrual pain (12). Lower AMH concentration has been
found in using oral contraceptives (13), mild/ minimal endometriosis (14), obesity (15), smoking (16), and a regular and shorter
menstrual cycle (13).
Physical
activity plays an important role in maintaining energy balance which may affect
the reproductive system (17). Weight loss via physical activity
may protect ovarian function by increasing insulin resistance and changing the
hormonal profile (18). It has been reported that an
increased risk of infertility was found for the group of women reporting the
highest levels of intensity and frequency of physical activity (19). Thus the
possible risks of infertility should be highlighted among women who do heavy
exercise. Steiner et al. (2010) reported that serum AMH levels do not fluctuate
during oral contraceptive use in reproductive-aged women and AMH levels are
significantly lower in obese women (1). It has been reported among
premenopausal women, that lower AMH levels are associated with older age,
younger age at menarche, and currently using oral contraceptives, suggesting
these factors are related to decreased ovarian follicles (20). Bernardi et al. (2017) reported a
significant association between obesity and lower AMH levels, suggesting that
obesity may compromise ovarian reserve(21) through decreased responses to
fertility medications, fewer oocytes retrieved (22), and lower pregnancy and live birth
rates (23). However, there are contradictions
in the literature regarding the association between obesity and AMH levels, so
further investigation into this relationship is warranted.
On
the other hand, ethnicity is an independent predictor for AMH (18) and the
association between AMH and lifestyle factors like body mass index (BMI),
smoking, and physical activity may vary across ethnic groups (13).
Understanding the factors associated with individual variation of AMH levels
among infertile women may help their infertility management. To our knowledge,
no study has targeted in North Iranian between infertile women to examine such
associations. Therefore, the present study aimed to evaluate the association
between age, BMI, reproductive history, and physical activity with serum AMH
concentration in North Iranian women with primary/secondary infertility.
Materials and Methods
Subjects
This cross-sectional study included 234 women aged 18–45 from April 2019 to March 2020. Patients participating in the study were selected from women
candidates for assisted reproductive treatment and referred to the Infertility
Clinic of the Al-Zahra Hospital, Rasht, Iran. Exclusion criteria included menopause,
cancer, underlying endocrine diseases, use of hormonal drugs, diagnosis of PCOS
based on Rotterdam criteria, any ovarian and uterine surgery, and
endometriosis. Approval was
obtained from the Research Deputy and Ethics Committee of Guilan University of
Medical Sciences (Approval ID: IR.GUMS.REC.1398.375). All the participants
signed a written informed consent before sample collection and acknowledged
that they had been fully anonymized. The reproductive characteristics included
age at menarche, cycle regularity status, pregnancy, parity, breastfeeding history, and age at menarche, maternal menopause age. The amount of
physical activity of the patients was also recorded. IPAQ (International Physical Activity Questionnaire) (24) was used to determine the amount of physical activity.
AMH assay
At the time of enrollment up to 5 mL of venous blood was drawn from
each participant. Blood samples were centrifuged at 1400g/10min to separate the
serum. Serum samples were stored at -20 °C until AMH concentration measurement.
Serum AMH was measured using the Beckman Coulter AMH ELISA kit (cat no: B13127)
according to the manufacturer’s instructions.
Statistical analysis
Statistical analyses were performed using SPSS Software (v21; SPSS
Inc; Chicago, Illinois, USA), and P-values less than 0.05 were considered
significant and Chi-square tests, fisher exact test, and independent T-test
were used to examine the relationship between variables
Results
This
cross-sectional analysis included 234 women aged 18–45 years old referred to
the Infertility Clinic of the Al-Zahra Hospital, Rasht, Iran. Table 1 includes
information on the demographics and reproductive history of the women who
participated in the study. As expected, we observed significantly lower AMH
concentrations in older participants. The risk of infertility is increased for
the group of women who report the highest intensity and frequency of physical
activity. There was no significant association
between BMI and AMH concentrations (P= 0.37). There was no association between
reproductive determinants and AMH level (Table 1).
Discussion
This
study demonstrated that AMH levels are influenced by physical activity. More
specifically, we found lack of physical activity, as well as vigorous physical
activity, is associated with lower AMH concentration. Improvement of AMH levels
and oxidative stress through regular exercise has been reported in Chinese
women with PCOS (25). So, improvement of oxidative
stress might be an effective method for improvement of AMH level, which
deserves further research. It has been reported that the level of AMH in
women over 40 years of age was significantly lower than in women less than 35
years of age. Jung et al.(2017) reported higher AMH concentrations in women with
older compared to younger ages at menarche (20) while our finding is consistent
with other study reported no associations (26). We also observed no association between other reproductive determinants
(Table 1) which may be due to the small sample size of the present study or
ethnicity variations. So, future large studies are warranted to validate our findings. We
observed no association between parity and AMH level that is consistent with earlier studies (20). The
decrease of AMH levels with increasing age in adult premenopausal women is well
established (26,27) as we observed in this present
study.
Regular
exercise causes weight loss and improves metabolic function and hormonal
profile. It has been reported that the exercises also usually lead to a
significant increase in fertility (28). Physical activity improves the
quality of life in the general population but there is insufficient evidence
for the effect of physical activity and quality of life on improving fertility
in infertile women (29). Cicek et al. (2019) reported
strength exercise decreases serum AMH levels and increases serum FSH levels (30). Therefore, excessive exercise
practices have negative consequences for women's fertility, especially for
those with lower ovarian reserve. It has been reported that moderate physical
activity is associated with improved age-specific levels of ovarian reserve
markers (31).
Physical
activity through regulation of energy balance and insulin sensitivity can
improve reproductive system function. Vigorous physical activity was associated
with reduced fecundity in all women with normal BMI, but not in overweight and
obese women (32). However, it has been demonstrated
physical activity is unlikely to have a deleterious effect on IVF success and
certain forms of vigorous activity may be beneficial (33). AMH can predict the ovarian
response to hyperstimulation (34) and a low AMH test result has a
negative psychological impact (35). On the other hand, maternal
lifestyle during pregnancy may be associated with reproductive health and
ovarian reserve in adult offspring (36). So, finding an association between
lifestyle parameters such as physical activity and the level of AMH, and
changing this lifestyle can affect the health of the next generation.
Conclusions
In this study, AMH levels appear to
be significantly lower in patients with a lack of physical activity as well as
vigorous physical activity. The results of the present study showed that by improving lifestyle,
including the appropriate amount of physical activity, it may be possible to
improve hormone levels and thus improve the results of infertility treatments. However, a larger clinical study is indicated to study the
association between AMH and physical activity in reproductive-age women.
Author contribution
In
this manuscript, the role of each of the authors, conceptualization with RK,
conceptualization with FM, data collection with ME, formal
Analysis with AA, writing, review and editing with NGhG, and writing
an original draft with SHSh.
Acknowledgments
The
authors appreciated Reproductive Health Research Center of Guilan University of
Medical Sciences for their constant support.
Conflict of interest
The
authors report no conflict of interest.
Funding/Support
This
Manuscript was not Funded.
References
1. Steiner AZ, Stanczyk FZ, Patel S, Edelman A.
Antimullerian hormone and obesity: insights in oral contraceptive users.
Contraception. 2010;81(3):245–8.
2. Karkanaki A, Vosnakis C, Panidis D. The
clinical significance of anti-Müllerian hormone evaluation in gynecological
endocrinology. Hormones. 2011;10(2):95–103.
3. Toner JP, Seifer DB. Why we may abandon
basal follicle-stimulating hormone testing: a sea change in determining ovarian
reserve using antimüllerian hormone. Fertil Steril. 2013;99(7):1825–30.
4. Dolleman M, Verschuren WMM, Eijkemans MJC,
Dollé MET, Jansen E, Broekmans FJM, et al. Reproductive and lifestyle
determinants of anti-Müllerian hormone in a large population-based study. J
Clin Endocrinol Metab. 2013;98(5):2106–15.
5. Kelsey TW, Wright P, Nelson SM, Anderson RA,
Wallace WHB. A validated model of serum anti-Müllerian hormone from conception
to menopause. PLoS One. 2011;6(7):e22024.
6. Sowers MFR, Eyvazzadeh AD, McConnell D,
Yosef M, Jannausch ML, Zhang D, et al. Anti-mullerian hormone and inhibin B in
the definition of ovarian aging and the menopause transition. J Clin Endocrinol
Metab. 2015;93(9):3478–83.
7. Iliodromiti S, Kelsey TW, Anderson RA,
Nelson SM. Can anti-Müllerian hormone predict the diagnosis of polycystic ovary
syndrome? A systematic review and meta-analysis of extracted data. J Clin
Endocrinol Metab. 2013;98(8):3332–40.
8. Knauff EAH, Eijkemans MJC, Lambalk CB, ten
Kate-Booij MJ, Hoek A, Beerendonk CCM, et al. Anti-Mullerian hormone, inhibin
B, and antral follicle count in young women with ovarian failure. J Clin
Endocrinol Metab. 2011;94(3):786–92.
9. Broer SL, Mol BWJ, Hendriks D, Broekmans
FJM. The role of antimullerian hormone in prediction of outcome after IVF:
comparison with the antral follicle count. Fertil Steril. 2011;91(3):705–14.
10. Broer SL, Eijkemans MJC, Scheffer GJ, Van
Rooij IAJ, De Vet A, Themmen APN, et al. Anti-Müllerian hormone predicts
menopause: a long-term follow-up study in normoovulatory women. J Clin
Endocrinol Metab. 2011;96(8):2532–9.
11. Nelson SM, Messow MC, Wallace AM, Fleming R,
McConnachie A. Nomogram for the decline in serum antimüllerian hormone: a
population study of 9,601 infertility patients. Fertil Steril.
2011;95(2):736–41.
12. Konishi S, Nishihama Y, Iida A, Yoshinaga J,
Imai H. Association of antimüllerian hormone levels with menstrual-cycle type
and dysmenorrhea in young asymptomatic women. Fertil Steril.
2014;102(5):1439–43.
13. Kristensen SL, Ramlau-Hansen CH, Andersen CY,
Ernst E, Olsen SF, Bonde JP, et al. The association between circulating levels
of antimüllerian hormone and follicle number, androgens, and menstrual cycle
characteristics in young women. Fertil Steril. 2012;97(3):779–85.
14. Lemos NA, Arbo E, Scalco R, Weiler E, Rosa V,
Cunha-Filho JS. Decreased anti-Müllerian hormone and altered ovarian follicular
cohort in infertile patients with mild/minimal endometriosis. Fertil Steril.
2011;89(5):1064–8.
15. Su HI, Sammel MD, Freeman EW, Lin H, DeBlasis
T, Gracia CR. Body size affects measures of ovarian reserve in late
reproductive age women. Menopause (New York, NY). 2015;15(5):857.
16. Sowers MR, McConnell D, Yosef M, Jannausch ML,
Harlow SD, Randolph Jr JF. Relating smoking, obesity, insulin resistance and
ovarian biomarker changes to the final menstrual period (FMP). Ann N Y Acad
Sci. 2010;1204:95.
17. Redman LM. Physical activity and its effects
on reproduction. Reprod Biomed Online. 201112(5):579–86.
18. Norman RJ, Noakes M, Wu R, Davies MJ, Moran L,
Wang JX. Improving reproductive performance in overweight/obese women with
effective weight management. Hum Reprod Update. 2014;10(3):267–80.
19. Gudmundsdottir
SL, Flanders WD, Augestad LB. Physical activity and fertility in women: The
North-Trøndelag Health Study. Hum Reprod. 2015;24(12):3196–204.
20. Jung S, Allen N, Arslan AA, Baglietto L,
Brinton LA, Egleston BL, et al. Demographic, lifestyle, and other factors in
relation to antimüllerian hormone levels in mostly late premenopausal women.
Fertil Steril. 2017;107(4):1012–22.
21. Bernardi LA, Carnethon MR, de Chavez PJ,
Ikhena DE, Neff LM, Baird DD, et al. Relationship between obesity and
anti‐Müllerian hormone in reproductive‐aged African American women. obesity.
2017;25(1):229–35.
22. Pinborg A, Gaarslev C, Hougaard CO, Andersen
AN, Andersen PK, Boivin J, et al. Influence of female bodyweight on IVF
outcome: a longitudinal multicentre cohort study of 487 infertile couples.
Reprod Biomed Online. 2011;23(4):490–9.
23. Shah DK, Missmer SA, Berry KF, Racowsky C,
Ginsburg ES. Effect of obesity on oocyte and embryo quality in women undergoing
in vitro fertilization. Obstet Gynecol. 2011;118(1):63–70.
24. Tomioka K, Iwamoto J, Saeki K, Okamoto N.
Reliability and validity of the International Physical Activity Questionnaire
(IPAQ) in elderly adults: the Fujiwara-kyo Study. J Epidemiol. 2011;1109210254.
25. Wu X, Wu H, Sun W, Wang C. Improvement of
anti-Müllerian hormone and oxidative stress through regular exercise in Chinese
women with polycystic ovary syndrome. Hormones. 2020;1–7.
26. Shaw CM, Stanczyk FZ, Egleston BL, Kahle LL,
Spittle CS, Godwin AK, et al. Serum antimüllerian hormone in healthy
premenopausal women. Fertil Steril. 2011;95(8):2718–21.
27. van Rooij IAJ, Broekmans FJM, Scheffer GJ,
Looman CWN, Habbema JDF, de Jong FH, et al. Serum antimüllerian hormone levels
best reflect the reproductive decline with age in normal women with proven
fertility: a longitudinal study. Fertil Steril. 2015;83(4):979–87.
28. Al-Eisa E, Gabr SA, Alghadir AH. Effects of
supervised aerobic training on the levels of anti-Mullerian hormone and
adiposity measures in women with normo-ovulatory and polycystic ovary syndrome.
J Pak Med Assoc. 2017;67(4):499–507.
29. Orio F, Muscogiuri G, Ascione A, Marciano F,
Volpe A, La Sala G, et al. Effects of physical exercise on the female
reproductive system. Minerva Endocrinol. 2013;38(3):305–19.
30. Cicek G, Gorkem U, Yamaner F, Gullu A, Gullu
E. Adverse Effect of Different Exercise Types on Ovarian Reserve. J Educ Train
Stud. 2019;7(1):115–20.
31. Kiranmayee D, Praveena T, Himabindu Y,
Sriharibabu M, Kavya K, Mahalakshmi M. The effect of moderate physical activity
on ovarian reserve markers in reproductive age women below and above 30 years.
J Hum Reprod Sci. 2017;10(1):44.
32. Wise LA, Rothman KJ, Mikkelsen EM, Sørensen
HT, Riis AH, Hatch EE. A prospective cohort study of physical activity and time
to pregnancy. Fertil Steril. 2012;97(5):1136–42.
33. Gaskins AJ, Williams PL, Keller MG, Souter I,
Hauser R, Chavarro JE, et al. Maternal physical and sedentary activities in
relation to reproductive outcomes following IVF. Reprod Biomed Online.
2016;33(4):513–21.
34. Dewailly D, Andersen CY, Balen A, Broekmans F,
Dilaver N, Fanchin R, et al. The physiology and clinical utility of
anti-Müllerian hormone in women. Hum Reprod Update. 2014;20(3):370–85.
35. O’Brien Y, Kelleher C, Wingfield M. “So what happens next?” exploring the psychological and
emotional impact of anti-Mullerian hormone testing. J Psychosom Obstet Gynecol.
2020;41(1):30–7.
36. Eubanks AA, Nobles CJ, Hill MJ, DeCherney AH,
Kim K, Sjaarda LA, et al. Recalled maternal lifestyle behaviors associated with
anti-müllerian hormone of adult female offspring. Reprod Toxicol. 2020;98:75–81.