Synchronous
primary malignancies of the lung and breast: a rare case report
Abeer Mundher Ali 1, Ahmed Dheyaa
Al-Obaidi 2, Mazin Judy Ibrahim 2, Mustafa
Najah Al-Obaidi 2, Muhammad Khuzzaim Khan 3*, Hashim
Talib Hashim 2
1 Al-Kadhimiya Teaching Hospital, Baghdad, Iraq
2 University of Baghdad, College of Medicine, Baghdad,
Iraq
2 Dow University of Health Sciences, Karachi, Pakistan
Corresponding Authors: Muhammad
Khuzzaim Khan
* Email: khuzzaimkhan@yahoo.com
Abstract
Introduction: Multiple Primary Malignant Tumors (MPMT) are two or more distinct
primary cancers in a single patient, either occurring simultaneously
(synchronous) or at different times (metachronous). MPMTs are very rare, with
an incidence of 0.73% to 11.7% among cancer patients. Breast and lung cancers
are the most common malignancies in women, but their coexistence as MPMT is
uncommon.
Case presentation: We report the case
of a 51-year-old non-smoking woman who had a productive cough with bloody
sputum for a week, after a two-month history of dry cough. She was diagnosed
with a high-grade, poorly differentiated non-keratinizing squamous-cell
carcinoma in the right lung. A PET scan also revealed a poorly defined soft
tissue mass in the central sector of the right breast, which was confirmed to
be a primary invasive ductal carcinoma.
Discussion: The etiology and pathogenesis of MPMT are unclear, but several factors
such as genetic predisposition, environmental exposure, immunodeficiency, and
treatment-related effects have been proposed. The diagnosis and management of
MPMT are challenging, as they require careful evaluation of each tumor and
individualized treatment plans. The prognosis of MPMT depends on the stage and
histology of each tumor, as well as the patient’s performance status and
comorbidities.
Conclusion: This case report highlights the rare occurrence of synchronous primary
malignancies in the lung and breast, underreported in the medical literature.
This case adds to the existing knowledge of MPMT and may stimulate further
research on this topic. Clinicians should be aware of the possibility of MPMT
in cancer patients and perform thorough investigations to rule out secondary or
metastatic tumors.
Keywords: Small cell carcinoma, Breast cancer, Synchronous, Metachronous,
Histopathology, Immunochemistry, Gene mutation
Introduction
Multiple
primary malignant tumors (MPMT) are two or more separate primary cancers in one
patient. They can be synchronous (discovered within six months) or metachronous
(discovered after six months). MPMT are very rare, affecting 0.73% to 11.70% of
cancer patients (1). Small-cell lung cancer (SCLC) and invasive ductal
carcinoma (IDC) are the most common cancers in women, but their coexistence as
MPMT is uncommon. SCLC is a fast-growing and aggressive lung cancer linked to
smoking (2). IDC is the most frequent type of breast cancer, making up 75% of
all cases (3). Usually, when both cancers are found, one is a metastasis from
the other. Chest X-rays and CT scans are used to diagnose lung metastases from
breast cancer or primary lung tumors (4). However, our case report describes a
rare situation: a patient with both breast cancer and primary lung cancer
detected by PET scan. The breast cancer was confirmed to be a separate primary
tumor. This unusual case challenges the conventional understanding and
highlights the complexity of MPMT. The purpose of this article is to report
this rare case and contribute to the existing knowledge and research on MPMT.
Case presentation
A
51-year-old female, a non-smoker, presented with a distressing clinical
profile. She experienced a productive cough accompanied by bloody sputum for
one week. This was preceded by a two-month history of dry cough, which
coincided with the onset of gradually increasing shortness of breath, notable
fatigue, decreased appetite, and significant weight loss. Her weight had
declined from 67 kg to 58 kg within three months. She denied any chest pain or
fever. Her medical history, surgical history, and family history did not reveal
any predisposition to cancer.
Initial
evaluation included a chest X-ray that revealed severe right-sided pleural
effusion. Subsequently, a contrast-enhanced chest CT scan depicted moderate
right-sided pleural effusion (as depicted in Figures 1A and 1B). The scan also
unveiled complete occlusion of the bronchus intermedius, along with total
collapse of the right middle lobe (Figure 1C). Additionally, the right upper
lobe exhibited interlobular septal thickening, and the right lower lobe
displayed partial collapse accompanied by extensive fibrotic changes (figure
1D). While no definitive obstructive mass was evident, the presentation
prompted further investigation through bronchoscopy.
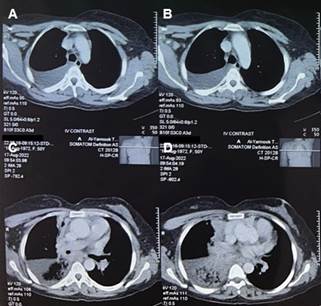
Figure 1. chest CT scan findings. Chest CT
scan with IV contrast displaying distinct aspects of the patient's condition: A
and B: Moderate right-sided pleural effusion. C: Total occlusion of the
bronchus intermedius along with complete collapse of the right middle lobe. D:
Interlobular septal thickening in the right upper lobe, accompanied by partial
collapse of the right lower lobe, revealing extensive fibrotic changes.
Bronchoscopy,
conducted under local anesthesia, revealed partial occlusion of the bronchus
intermedius, attributed to mass effect, leading to obstruction of the middle
and lower lung lobes. Subsequent lung biopsy disclosed a histopathological
profile consistent with high-grade, poorly differentiated non-keratinizing
squamous-cell carcinoma. Noteworthy characteristics included tumor infiltration
in single and solid sheets with dense desmoplastic fibrosis, limited
lymphocytic infiltration between tumor cells, and absence of stromal
lymphovascular and peri-neural tumor involvement. Immunohistochemistry
confirmed positive cytokeratin 5/6.
Further
investigations were carried out to assess metastatic spread. A PET scan
revealed Fluorodeoxyglucose (FDG) uptake in several regions. An ill-defined
mass lesion in the right lung hilum (6.14.5 cm) exhibited maximum standardized
uptake value (SUVmax) of 19.2 (Figure 2A). FDG uptake was also noted in
prevascular lymph nodes, bilateral paratracheal, right hilar, and subcarinal
(Figure 2B, 2D). The largest node measured 3.11.7 cm with SUVmax 4.4.
Additionally, a poorly defined soft tissue dense lesion (1.5*0.8 cm) in the
central sector of the right breast displayed SUVmax of 5.9 (figure 2C). A
smaller, benign-appearing nodule was observed in the upper inner quadrant of
the right breast (Figure 2C).
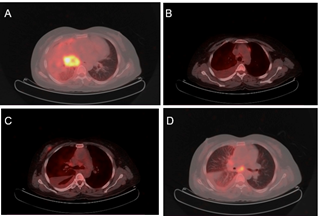
Figure 2. FDG-PET scan results. FDG-PET scan
images showcasing notable observations: A: Reveals FDG uptake in an ill-defined
mass lesion located in the hilar region of the right lung, involving bilateral
paratracheal and right hilar lymph nodes. B: Illustrates FDG uptake in the
prevascular lymph nodes. C: Demonstrates FDG uptake in an unwell-defined, dense
soft tissue mass lesion in the central sector of the right breast.
Additionally, it reveals the absence of significant FDG uptake in a smaller,
dense soft tissue nodule located in the upper inner quadrant of the right
breast. D: Displays FDG uptake in the subcarinal lymph nodes.
Ultrasonography of the right breast revealed an irregular
ill-defined hypoechoic mass (16.6*10mm) within the subareolar region, along
with internal echogenic foci (calcifications) classifying it as BI-RAD score
four (Figure 3).
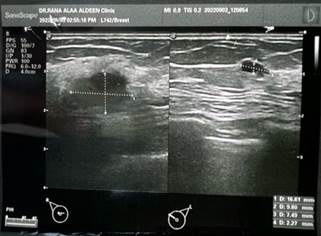
Figure 3. Breast ultrasonography findings. Breast ultrasonography image
presenting specific features of interest: This image demonstrates: An irregular
and ill-defined hypoechoic mass in the right breast, measuring 16.6 mm by 10
mm. The presence of internal tiny echogenic foci, indicative of calcifications.
Notably, the mass is situated within the subareolar region.
Subsequent fine-needle aspiration (FNA) confirmed malignant
features, displaying hyperchromatic irregular nuclei in clusters and scattered
epithelial cells within a necrotic background (Figures 4A and 4B).
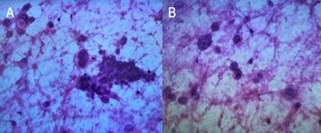
Figure 4. Cytological smears with H&E staining. Cytological smears
stained with H&E, highlighting specific cellular characteristics: A and B:
Depict clusters and scattered malignant epithelial cells, featuring
hyperchromatic irregular nuclear borders and pleomorphic nuclei. These cells
are set against a necrotic background.
Utilizing
ultrasound guidance, a cell-block preparation of the right breast mass
exhibited hyperchromatic and pleomorphic malignant cells with
intermediate-grade nuclear atypia. Focal tubular differentiation and
infiltration into fatty tissue were evident (Figures 5A and 5B). These findings
corresponded to invasive ductal carcinoma (not otherwise specified), supported
by immunohistochemistry results including positive CK7 and negative p63.
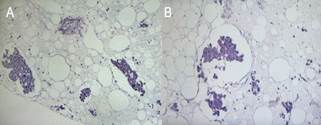
Figure 5. Cell-block preparation with H&E
staining.
Cell-block preparation stained with H&E, emphasizing
distinctive cellular attributes: A and B: Display hyperchromatic and
pleomorphic malignant cells exhibiting intermediate-grade nuclear atypia.
Notably, a focal region of tubular differentiation is also evident within the
preparation.
Slide
review and immunohistochemistry of the lung biopsy highlighted scattered
malignant cells intermingled with inflammatory cells. Positive CK7 and absence
of p63 confirmed epithelial origin, excluding lymphoma and small-cell
carcinoma. The overall histopathology and immunohistochemistry aligned with
poorly differentiated carcinoma exhibiting positive ER (3+5 8/8) and HER2
(score +3).
Collectively,
these findings indicated the presence of two distinct cancerous lesions, each
originating in different tissue types, with no evidence of metastasis between
sites. The patient was eligible for palliative chemotherapy with trastuzumab
and carboplatin, targeting both lung and breast cancers. Trastuzumab is a
monoclonal antibody that binds to the HER2 protein, which is overexpressed in
some cancers, and blocks its activity and triggers immune reactions that kill
cancer cells. Carboplatin is a platinum-based drug that damages the DNA of
cancer cells and prevents them from dividing. This combination is effective and
well-tolerated in patients with HER2-positive breast cancer and may also have
activity in HER2-positive lung cancer. The patient received an 8 mg/kg loading
dose of trastuzumab followed by 6 mg/kg every three weeks, along with 5 mg/kg
carboplatin every three weeks for at least six cycles. The expected outcomes of
this treatment were to control the disease progression, reduce the tumor
burden, and improve the quality of life. The potential side effects of this
treatment included nausea, vomiting, fatigue, hair loss, low blood counts,
infection, allergic reaction, kidney damage, nerve damage, and heart damage.
The patient was monitored for these side effects and received supportive care
as needed.
Discussion
Multiple
primary malignant tumors (MPMT) are becoming more recognized due to improved
diagnostic methods. However, diagnosing multiple primary lung cancers is still
challenging, especially when they have the same histology. Gene mutation
analysis can help to differentiate between primary and metastatic tumors (5).
Synchronous breast and lung cancers are very rare, accounting for less than
0.5% of breast cancer cases. A study by Burstein et al. showed that 55% of lung
lesions in women with breast cancer were primary lung cancers, 37% were
metastases, and 8% were benign (6). This highlights the need for accurate
histological diagnosis of lung lesions, as some of them may be treatable. This
also follows the criteria by Warren and Gates for diagnosing MPMT, which
require biopsy confirmation, distinct pathology, and exclusion of metastasis (6).
De Luca et al. reported a case of synchronous skin and breast cancer and
discussed the frequent co-occurrence of dual primary breast and lung cancers.
They attributed this to three factors: the high prevalence of breast cancer in
women, the good prognosis of early-detected breast cancer leading to an increased
risk of secondary tumors, and the increased susceptibility of breast cancer
survivors to develop primary lung tumors (7). Jin et al. described another rare
case of a woman with lesions in the left breast and both lower lung lobes. They
found that the lung lesions had different EGFR gene mutations, indicating
genetic heterogeneity among primary malignancies (8). Hu et al. also studied
the relationship between breast and lung cancers and found a strong correlation
between EGFR mutation in lung cancer and hormone receptor expression in lung
tissue. However, they did not find any association between EGFR mutation and
HER2 expression, suggesting a possible role of sex hormones in lung cancer
development in these patients (9). Besides breast and lung cancers, patients
with breast cancer may also develop primary tumors in other organs, such as the
ovaries, uterus/endometrium, colorectum, kidneys, pancreas, and thyroid. These
occurrences may manifest synchronously or metachronously, often influenced by
factors such as hormonal treatment for the primary breast tumor (notably, a
strong link exists between endometrial cancer and tamoxifen), genetic
predispositions (e.g., BRCA1 and BRCA2 mutations), and obesity. Incidence rates
fluctuate, with reported figures ranging from 4.1% by Kim and Song in a study
tracking 108 breast cancer patients, to 16.4% by Weir et al., who followed a larger
cohort of 301,963 patients (10). However, the convergence of multiple distinct
cancer types, exemplified by the case presented here, remains a rare
phenomenon.
This
case report presents a rare occurrence of synchronous primary malignancies in
both the lung and breast, which is underreported in the medical literature.
This case adds to the existing knowledge of MPMT and may stimulate further
research on this topic. Future directions for research may include genetic
profiling, targeted therapies, or novel treatment approaches that could improve
our understanding and management of these rare synchronous malignancies.
Our
study has some limitations that should be considered when interpreting our
results. First, our sample size was small, consisting of only one patient with
synchronous primary malignancies in both the lung and breast. Therefore, our
findings may not be generalizable to other patients with similar conditions.
Second, our study was a case report, which is a descriptive and observational
type of study that does not provide causal evidence or test hypotheses.
Therefore, our study cannot establish the etiology, pathogenesis, or prognosis
of these rare synchronous malignancies. Further studies with larger and more
diverse samples are needed to confirm and expand our findings.
Author contribution
MKK, MJI,
and AMA contributed to the conception and design of the manuscript. MKK,
MJI, HTH, and AMA supervised the project. MKK, MJI,
MNA, HTH, and ADA provided the materials and contributed
to data collection and processing. ADA, MJI, MKK, MNA,
HTH and AMA contributed to the interpretation and analysis of the
project. ADA, HTH, and MNA contributed to the literature
review and writing of the manuscript respectively. ADA, MNA and AMA
critically revised the manuscript.
IRB approval
The
case report was approved for publication by the University of Baghdad’s
Institutional Review Board under the ethics code UB/2023/022.
Ethics Statement
The
manuscript complies with the ethical recommendations of the Declaration of
Helsinki of the World Medical Association
Conflict of interest
The
authors declare no conflict of interest.
Funding/Support
No institutional or financial
support was received.
References
1. Jin B, Zhang S, Chuang X, Yu P, Chen Y, Teng
Y, et al. Breast cancer and synchronous multiple primary lung adenocarcinomas
with heterogeneous mutations: A case report. BMC Cancer. 2018;18(1):2–7.
2. Holly A. Swartz, Jessica C. Levenson and EFU. HHS Public Access Small-cell lung
cancer. Physiol Behav. 2012;43(2):145–53.
3. Waks AG, Winer EP. Breast Cancer Treatment:
A Review. JAMA. 2019;321(3):288-300. doi:10.1001/jama.2018.19323
4. Paul Spiteri Meilak B. The relationship
between primary breast and primary lung cancer: A case series. Int J Case
Reports Images. 2020;11(July):1.
5. Lekos A, Glantz MJ. Diagnosis in oncology. J
Clin Oncol. 1997;15(8):3019–20.
6. De Luca A, Frusone F, Vergine M, Cocchiara
R, Torre G La, Ballesio L, et al. Breast cancer and multiple primary malignant
tumors: Case report and review of the literature. In Vivo (Brooklyn).
2019;33(4):1313–24.
7. Hu Z, Zou X, Qin S, Li Y, Wang H, Yu H, et
al. Hormone receptor expression correlates with EGFR gene mutation in lung
cancer in patients with simultaneous primary breast cancer. Transl Lung Cancer
Res. 2020;9(2):325–36.
8. Vogt A, Schmid S, Heinimann K, Frick H,
Herrmann C, Cerny T, et al. Multiple primary tumours: Challenges and
approaches, a review. ESMO Open. 2017;2(2):1–12.
9. Kim JY, Song HS. Metachronous double primary
cancer after treatment of breast cancer. Cancer Res Treat. 2015;47(1):64–71.
10.
Weir HK, Johnson CJ, Thompson TD. The effect of multiple primary
rules on population-based cancer survival. Cancer Causes Control.
2013;24(6):1231–42.