A practical
general review of lung cancer
Morteza Pourqasemi 1, Roshanak Ale-Esmaiel
2, Tofigh Yaghubi-Kalurazi 3*
1 Counseling and anti-tuberculosis Center, Razi
Hospital, Guilan University of Medical Sciences, Rasht, Iran
2 Radiology and Nuclear Medicine Department, School of
Paramedical Sciences, Kermanshah University of Medical Sciences, Kermanshah,
Iran
3 Department of Health, Nutrition & Infectious
Diseases, School of Medicine, Guilan University of Medical Sciences, Rasht,
Iran
Corresponding Authors: Tofigh
Yaghubi-Kalurazi
* Email: tofigh_yaghubi@yahoo.com
Abstract
Lung cancer, also known as lung carcinoma, is a malignant tumor
that begins in the lung. Lung cancer is caused by genetic damage to the DNA of
cells in the airways and is often caused by cigarette smoking or inhalation of
harmful chemicals. Damaged airway cells gain the ability to multiply unchecked,
causing tumor growth. Without treatment, tumors spread throughout the lungs,
damaging lung function. Eventually, the lung tumors metastasize and spread to
other body parts. On the other hand, lung cancer or bronchogenic carcinoma
refers to tumors originating in the lung parenchyma or within the bronchi. It
ranks among the primary causes of cancer-related mortality globally. It is
estimated that there is an increasing rate of new cases of lung cancer
worldwide annually, with an approximately high mortality rate because of lung
cancer. It is worth mentioning that lung cancer was a relatively uncommon
condition at the beginning of the 20th century. Its dramatic rise in later
decades is primarily attributable to the increase in smoking among both males
and females. Treatments include surgery, chemotherapy, immunotherapy,
radiation, and targeted drugs. This review article describes lung cancer's
causes, pathophysiology, and presentation.
Keywords: Lung cancer, Etiology, Diagnosis, Treatment
Introduction
Lung
cancer, also known as bronchogenic carcinoma, denotes the development of tumors
within the lung parenchyma or bronchi. It stands as a prominent contributor to
cancer-related mortality in the United States. Since 1987, lung cancer has
surpassed breast cancer as the leading cause of death among women. Annually, an
estimated 225,000 new cases of lung cancer are diagnosed in the United States,
resulting in approximately 160,000 fatalities. Notably, lung cancer was a
relatively uncommon ailment at the onset of the 20th century, with its
substantial escalation in subsequent decades largely attributed to the
heightened prevalence of smoking among both genders (Figure 1) (1, 2).
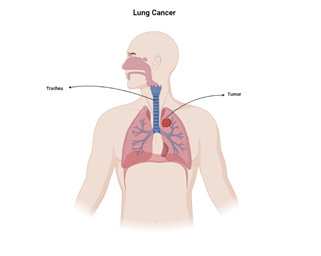
Figure 1. A schematic
picture of the location of lung cancer.
Etiology
The
predominant factor contributing to the development of lung cancer is smoking.
It is approximated that smoking accounts for 90% of lung cancer cases (3). The highest risk of developing
lung cancer is observed in male individuals who engage in smoking. This risk is
further exacerbated by exposure to additional carcinogens, such as asbestos.
The relationship between the incidence of lung cancer and the quantity of
cigarette packs smoked annually is not directly correlated, owing to the
intricate interaction between smoking habits and various environmental and
genetic influences. Additionally, the risk of developing lung cancer as a
result of passive smoking is augmented by 20 to 30% (3). Additional factors to consider are
the use of radiation therapy for the treatment of cancers other than lung
cancer, particularly non-Hodgkin's lymphoma and breast cancer (4). Exposure to certain metals,
including chromium, nickel, arsenic, and polycyclic aromatic
hydrocarbons, has been linked to an increased risk of lung cancer.
Additionally, lung diseases such as idiopathic pulmonary fibrosis can
independently raise the risk of lung cancer, regardless of smoking habits.
Asbestos and radon are well-established risk factors for lung cancer (5). The risk of lung cancer associated
with asbestos exposure, particularly in occupational settings, increases
proportionally with the dose and varies based on the type of asbestos fiber.
The risk from nonoccupational asbestos exposure is less clearly defined.
However, the United States Environmental Protection Agency (EPA) has
established standards for acceptable low-level nonoccupational asbestos
exposure. The EPA states that if asbestos is undisturbed and does not release
respirable particles, the health risk to occupants of a building is not
significant (6). Radon exposure in uranium miners
was associated with a small but significant risk of lung cancer (7). Radon has been demonstrated to
build up in residential environments as a byproduct of the decay of uranium and
radium. A comprehensive analysis of studies conducted in Europe revealed
significant risks associated with residential radon exposure, particularly for
individuals who smoke. This exposure was found to be accountable for
approximately 2% of all lung cancer-related deaths in Europe (8).
Epidemiology
Lung
cancer is the most frequently identified form of cancer on a global scale,
constituting around 12.4% of all cancer diagnoses worldwide, and stands as the
primary contributor to cancer-related mortality (9). The American Cancer Society
projects that there will be more than 234,000 new cases of lung cancer and over
154,000 deaths associated with lung cancer in the United States annually (9). Based on the 2020 Global Cancer
Statistics report, it was found that lung cancer continued to be the primary
contributor to global cancer-related mortality, resulting in approximately 1.8
million deaths (10). In the past, the prevalence of
lung cancer appeared to primarily affect developed nations. However, recent
evidence indicates a significant increase in lung cancer incidence, with nearly
half of new cases, 49.9%, being diagnosed in underdeveloped regions(11). In the United States, there is a
higher mortality rate among men compared to women. While there is no racial
disparity in the occurrence of lung cancer overall, the age-adjusted mortality
rate is elevated in African-American males in comparison to Caucasian males.
This distinction is not observed among women (3).
Pathophysiology
The
pathophysiology of lung cancer is a multifaceted and not fully elucidated
process. It is postulated that recurrent exposure to carcinogens, particularly
from cigarette smoke, results in the development of dysplasia in the lung
epithelium. Prolonged exposure further leads to genetic mutations and disrupts
protein synthesis (12). This consequently interrupts the
process of cell division and encourages the formation of cancer. The prevalent
genetic alterations associated with the onset of lung cancer include MYC, BCL2,
and p53 for small cell lung cancer (SCLC), and EGFR, KRAS, and p16 for
non-small cell lung cancer (NSCLC) (13, 14). The
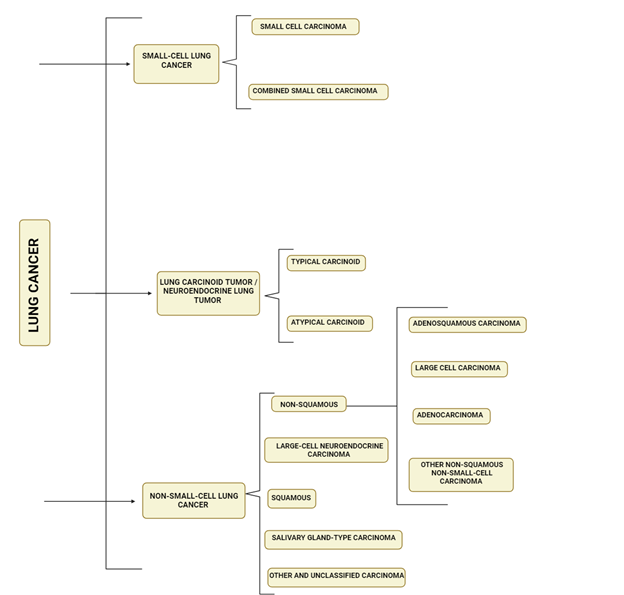
histopathological categorization of lung cancers is crucial for their diagnosis
and management, and is based on cellular and molecular subtypes. The 2021 World
Health Organization (WHO) classification system divides lung cancers into
various categories, including precursor glandular lesions, adenocarcinomas,
adenosquamous carcinomas, squamous precursor lesions, squamous cell carcinomas,
large cell carcinomas, sarcomatoid
carcinomas, lung neuroendocrine neoplasms,
salivary gland-type tumors, neuroendocrine tumors, neuroendocrine carcinomas,
and other epithelial tumors. The WHO emphasizes the identification of
histologic features, measurement of invasion depth, and mode of spread for
prognostic purposes. For instance, the presence of tumor spread through air
spaces is associated with a higher recurrence rate after limited resections and
should be documented in pathological evaluations. Additionally, the WHO has
discontinued the clear cell, rhabdoid, and signet ring subtypes in the most
recent classification, as they are considered to be cytologic features that can
occur in any adenocarcinoma. The WHO classification system places significant
emphasis on the use of immunohistochemical staining to classify cancers that
may not exhibit typical cytologic features under light microscopy (Figure
2). In the 2015 classification system established by the World Health
Organization (WHO), poorly differentiated carcinomas underwent reclassification
based on specific biomarker expressions. Those exhibiting p40 expression were
reclassified as squamous cell carcinomas, while those demonstrating thyroid
transcription factor 1 expression were categorized as adenocarcinomas with
solid subtype. Additionally, carcinomas showing positivity for chromogranin and
synaptophysin were reclassified as neuroendocrine carcinomas.
Precursor
Glandular Lesions
These
lesions encompass atypical adenomatous hyperplasia (AAH) and adenocarcinoma in
situ. AAH serves as a precursor to lung adenocarcinoma and typically presents
as a lesion measuring ≤ 5 mm. Adenocarcinoma in situ can manifest as either
mucinous or nonmucinous and is generally a localized lesion of 3 cm or less. It
exhibits a "lepidic" growth pattern, characterized by growth confined
along the alveolar structures. This type of lesion is non-invasive and
demonstrates intact alveolar septa.
Adenocarcinoma
The
pathology of adenocarcinoma involves the development of neoplastic gland
formation and the expression of pneumocyte markers such as thyroid
transcription factor 1 (TTF-1) with or without napsin expression, or
intracytoplasmic mucin. It is further categorized based on the extent and
structure of neoplastic gland formation as either mucinous or non-mucinous. The
non-mucinous subtypes include acinar, papillary, micropapillary, lepidic, and
solid subtypes. Accurate pathological identification of these subtypes is
crucial for determining prognosis. Specifically, the solid, micropapillary, and
cribriform (a subtype of acinar non-mucinous adenocarcinoma) patterns are
associated with unfavorable prognostic implications (15). Mucinous adenocarcinomas may
exhibit various architectural patterns such as papillary, micropapillary,
solid, and cribriform. However, the World Health Organization (WHO) does not
provide grading recommendations for mucinous carcinomas based on these growth
patterns. Other less common forms of adenocarcinoma include colloid,
enteric-like, lymphoepithelial, and fetal forms. Minimally invasive
adenocarcinoma (MIA) is characterized by a small, solitary adenocarcinoma
measuring ≤ 3 cm with minimal invasion (less than 5 mm) and a predominant
lepidic growth pattern, resembling similar precursor glandular lesions. If the
invasion exceeds 5 mm, it is classified as lepidic-predominant adenocarcinoma.
Invasive mucinous adenocarcinoma, previously known as mucinous bronchioloalveolar
carcinoma, encompasses mucinous lesions that do not meet the criteria for MIA.
Lesions with more than 10% of mucinous and non-mucinous growth patterns should
be classified as mixed adenocarcinoma.
Chemotherapy
Chemotherapy
remains a primary treatment option for patients who are not suitable candidates
for single-agent immunotherapy or combination immunotherapy regimens. These
patients may have contraindications to immunotherapy, such as pre-existing
autoimmune conditions, or there may be concerns about their performance status
and the potential for toxicity with combination immunotherapy regimens. In such
cases, platinum doublets are commonly utilized. For patients with nonsquamous
metastatic NSCLC, a typical example would involve the use of carboplatin or
cisplatin in combination with pemetrexed for 4–6 cycles, followed by
maintenance pemetrexed until disease progression or unacceptable toxicity. In
the case of squamous metastatic NSCLC, a platinum doublet may consist of
carboplatin or cisplatin in combination with either paclitaxel or gemcitabine.
Biomarker
testing
Tailoring
medical treatment by focusing on specific molecular targets within tumors has
led to enhanced survival rates for individuals with non-small cell lung cancer
(NSCLC) (55). Various specific drugs have
demonstrated efficacy in treating mutations in the epidermal growth factor
receptor (EGFR) and anaplastic lymphoma kinase (ALK). Genomic testing has
identified additional molecular alterations such as ROS1 and RET gene rearrangements,
MET amplification, and activating mutations in BRAF, HER2, and KRAS genes.
These findings suggest potential targets for future therapeutic interventions.
Epidermal
growth factor receptor (EGFR) gene
The
EGFR receptor is a tyrosine kinase receptor located on the surface of cells,
capable of initiating signaling pathways related to cellular growth and
proliferation upon activation. In the context of cancer, mutations in the EGFR
gene result in unregulated cell division due to continuous activation. These
mutations are observed in 10-15% of lung cancer adenocarcinoma patients of
European and Asian ancestry, particularly in individuals who have never smoked
and in females (56-58). Although
these traits are prevalent, mutation testing plays a crucial role in
identifying individuals who would gain from targeted tyrosine kinase inhibitor
treatment. Mutations in EGFR commonly arise in exons 18–21, which confer
sensitivity to EGFR tyrosine kinase inhibitors; these exons encode a segment of
the EGFR kinase domain. Roughly 90% of these mutations consist of exon 19
deletions and the L858R point mutation on exon 21, and are associated with a
70% response rate in patients undergoing erlotinib or gefitinib therapy (59).
KRAS
The
KRAS oncogene is frequently mutated in non-small cell lung cancer (NSCLC)
through missense mutations that result in the substitution of an amino acid at
positions 12, 13, or 61. Mutations at residues G12 and G13 are particularly
prevalent. These mutations are more commonly found in adenocarcinomas,
individuals of Caucasian descent, and those with a history of smoking (60). Roughly 10 to 25% of individuals
diagnosed with adenocarcinoma exhibit tumors that are associated with KRAS
mutations (61). In the context of concurrent
occurrence with other cancer-causing genetic mutations, KRAS has been primarily
identified in tumor types that lack mutations in EGFR and ALK, indicating that
these mutations represent a distinct molecular subset of non-small cell lung
cancer (NSCLC). Recent evidence indicates that KRAS mutations may have
potential prognostic significance, but their ability to predict the response to
EGFR tyrosine kinase inhibitors or cytotoxic chemotherapy is limited (55, 59). A study has proposed the
feasibility of specifically targeting a subset of KRAS mutations using
small-molecule inhibitors designed to address the prevalent G12C mutation in
lung cancer, which is more common in smokers than non-smokers. These potential new
agents depend on binding to the mutant cysteine and do not impact the wild-type
KRAS protein, demonstrating specificity for a particular subtype (62).
Anaplastic
lymphoma kinase (ALK)
Roughly
3-7% of lung tumors exhibit ALK mutations, (63-65) which are
frequently observed in younger patients. Koh et al. found that individuals with
ALK mutations had a median age of 49, while those without ALK mutations had a
median age of 61 (P<0.001; n=221) (66). ALK mutations are also prevalent
in adenocarcinoma patients with acinar histology or signet ring cells, as well
as in those who have no history of smoking (67, 68). The
predominant ALK rearrangement observed in non-small cell lung cancer (NSCLC)
patients is the EML-4-ALK rearrangement. This genetic alteration occurs on
chromosome 2p23 and involves the fusion of the 5' end of the EML-4 gene with
the 3' end of the ALK gene, resulting in at least nine distinct fusion
variants. EML-4 mutations are frequently identified in adenocarcinomas of
individuals with no history of smoking or light smoking, whose tumors do not
exhibit mutations in either EGFR or KRAS genes (63, 68). ALK mutations
do not overlap with other oncogenic mutations linked to non-small cell lung
cancer, such as EGFR or RAS mutations (68, 69). Additional
ALK mutations unrelated to EML-4, such as KIF5B-ALK and TFG-ALK, have been
identified. Patients with EML4-ALK fusions or ALK rearrangements do not derive
therapeutic benefits from EGFR-specific tyrosine kinase inhibitor therapy (70).
Presently,
there exists an FDA-approved medication, crizotinib (Xalkori®, Pfizer), which
is designed to target constitutively activated receptor tyrosine kinases
resulting from EML4-ALK and other ALK fusions. A single arm study of
ALK-positive metastatic NSCLC(71), demonstrated objective response
rates of 50–61% in patients. In a trial involving previously untreated advanced
non-squamous ALK-positive NSCLC, patients were randomly assigned to receive
either crizotinib 250 mg orally twice daily (n=172) or intravenous chemotherapy
(pemetrexed 500 mg/m2 plus either cisplatin 75 mg/m2 or carboplatin target area
under the curve 5–6 mg/mL/min (PPC group); all administered intravenously every
three weeks for ≤6 cycles, n=171). The primary endpoint of the study was progression-free
survival, while secondary endpoints included overall response rate, overall
survival, safety, and patient-reported outcomes. The study revealed that
crizotinib extended progression-free survival to 10.9 months compared to 7
months in patients receiving PPC. Additionally, the overall response rate was
higher in patients receiving crizotinib at 74% compared to 45% in patients
receiving PPC. Overall, crizotinib demonstrated significant improvements in
progression-free survival and overall response rate compared to standard
chemotherapy, and its safety profile was deemed acceptable (71). This landmark study solidified
crizotinib as the recommended treatment for individuals with advanced
ALK-positive non-squamous non-small cell lung cancer who have not received
prior therapy.
BRAF
The
BRAF gene is classified as a proto-oncogene, functioning as a controlled signal
transduction serine/threonine protein kinase that has the capability to
stimulate cell proliferation and viability (72). Somatic mutations in the BRAF gene
have been identified in 1–4% of non-small cell lung cancer (NSCLC) cases, with
the highest prevalence observed in patients diagnosed with adenocarcinomas (61, 73-77). These
mutations are frequently associated with individuals who have a history of
smoking, either currently or in the past (76, 77). The localization of BRAF mutations
within the kinase domain varies between lung cancer and breast cancer patients.
A study involving 697 individuals diagnosed with lung adenocarcinoma revealed
that 3% of the patients harbored BRAF mutations, with the identified mutations
being V600E (50%), G469A (39%), and D594G (11%) (76). The majority of BRAF mutations in
non-small cell lung cancer (NSCLC) have been identified as distinct from other
oncogenic mutations, such as EGFR mutations and ALK rearrangements.
Conclusion
Lung
cancer is the primary contributor to cancer-related fatalities on a global
scale, resulting in the highest mortality rates for both genders. Approximately
85% of lung cancer cases are attributed to smoking. Diagnosis of lung cancer
frequently occurs at advanced stages, limiting treatment options. Screening
individuals at high risk has the potential to facilitate early detection and
significantly enhance survival rates. Implementing primary prevention
strategies, such as tobacco control measures and minimizing exposure to
environmental risk factors, has the potential to decrease the occurrence of
lung cancer and ultimately save lives. Considerable progress has been achieved
in mitigating occupational health risks related to lung cancer, particularly in
the context of smoking, and in the prevention of diverse disorders. In recent
years, targeted therapy and immunotherapy have significantly contributed to the
enhanced management of lung cancer. Furthermore, genetic and biomarker testing
are aiding in the personalized management of different types of lung cancer.
Through personalized management of non-small cell lung cancer (NSCLC),
treatments are tailored to individual patients and can specifically target
mutations with greater precision, aiming to prolong progression-free survival.
Immunotherapy involves the concept of enhancing and directing the body's own
immune defenses to combat cancer cells. Ongoing clinical trials are exploring
the use of vaccines for treating NSCLC. Given that lung cancer is the leading
cause of cancer-related deaths in the United States, ongoing research efforts
are focused on developing innovative treatments.
In
the past ten years, the landscape of lung cancer treatment in Canada has
experienced rapid changes. New targets have been identified, leading to
significant benefits for patients with metastatic non-small cell lung cancer
(NSCLC), particularly those without a history of smoking. The integration of
immunotherapy has altered the standard of care for patients with metastatic
NSCLC and is now being incorporated into earlier stages of treatment.
Physicians treating lung cancer must now be able to identify and manage the
specific toxicities associated with immunotherapy. This review has only
addressed some of the complexities involved in treating NSCLC and has not
delved into the details of therapy sequencing. Despite these advancements, lung
cancer continues to impose a substantial burden of morbidity and mortality on
the Canadian population. Smoking cessation and screening high-risk individuals
are crucial strategies for alleviating this burden.
Author contribution
MP conceptualized
and wrote the manuscript. TYK edited the final version of the
manuscript. RAE accompanied in writing of some sections of the paper.
All authors have read and confirmed the final revised version of the
manuscript.
Conflict of interest
The authors declare no
conflict of interest.
Acknowledgments
We express our
deep appreciation to all the people who contributed to this narrative review article.
References
1. Miller
KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer
treatment and survivorship statistics, 2016. CA: a cancer journal for
clinicians. 2016;66(4):271-89.
2. Kocher F, Hilbe W, Seeber A, Pircher A,
Schmid T, Greil R, et al. Longitudinal analysis of 2293 NSCLC patients: a
comprehensive study from the TYROL registry. Lung Cancer. 2015;87(2):193-200.
3. Alberg AJ, Samet JM. Epidemiology of lung
cancer. Chest. 2003;123(1):21S-49S.
4. Lorigan P, Radford J, Howell A, Thatcher N.
Lung cancer after treatment for Hodgkin's lymphoma: a systematic review. The
lancet oncology. 2005;6(10):773-9.
5. Burns DM. Primary prevention, smoking, and
smoking cessation: implications for future trends in lung cancer prevention.
Cancer. 2000;89(S11):2506-9.
6. Wagner GR. Asbestosis and silicosis. The
Lancet. 1997;349(9061):1311-5.
7. Grosche B, Kreuzer M, Kreisheimer M,
Schnelzer M, Tschense A. Lung cancer risk among German male uranium miners: a
cohort study, 1946–1998. British journal of cancer. 2006;95(9):1280-7.
8. Darby S, Hill D, Auvinen A, Barros-Dios J,
Baysson H, Bochicchio F, et al. Radon in homes and risk of lung cancer:
collaborative analysis of individual data from 13 European case-control
studies. Bmj. 2005;330(7485):223.
9. Siegel RL, Miller KD, Jemal A. Cancer
statistics, 2019. CA: a cancer journal for clinicians. 2019;69(1):7-34.
10. Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in 185 countries.
CA: a cancer journal for clinicians. 2021;71(3):209-49.
11. Barta JA, Powell CA, Wisnivesky JP. Global
epidemiology of lung cancer. Annals of global health. 2019;85(1).
12. Cagle PT, Allen TC, Olsen RJ. Lung cancer
biomarkers: present status and future developments. Archives of Pathology and
Laboratory medicine. 2013;137(9):1191-8.
13. Lindeman NI, Cagle PT, Beasley MB, Chitale
DA, Dacic S, Giaccone G, et al. Molecular testing guideline for selection of
lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline
from the College of American Pathologists, International Association for the
Study of Lung Cancer, and Association for Molecular Pathology. Journal of
Thoracic Oncology. 2013;8(7):823-59.
14. Lindeman NI, Cagle PT, Aisner DL, Arcila ME,
Beasley MB, Bernicker EH, et al. Updated molecular testing guideline for the
selection of lung cancer patients for treatment with targeted tyrosine kinase
inhibitors: guideline from the College of American Pathologists, the
International Association for the Study of Lung Cancer, and the Association for
Molecular Pathology. Archives of pathology & laboratory medicine.
2018;142(3):321-46.
15. Kadota K, Yeh Y-C, Sima CS, Rusch VW, Moreira
AL, Adusumilli PS, et al. The cribriform pattern identifies a subset of acinar
predominant tumors with poor prognosis in patients with stage I lung
adenocarcinoma: a conceptual proposal to classify cribriform predominant tumors
as a distinct histologic subtype. Modern Pathology. 2014;27(5):690-700.
16. Filosso PL, Ruffini E, Asioli S, Giobbe R,
Macri L, Bruna MC, et al. Adenosquamous lung carcinomas: a histologic subtype
with poor prognosis. Lung cancer. 2011;74(1):25-9.
17. Rajdev K, Siddiqui AH, Ibrahim U, Patibandla
P, Khan T, El-Sayegh D, et al. An unusually aggressive large cell carcinoma of
the lung: undiagnosed until autopsy. Cureus. 2018;10(2).
18. Aisner SC, Finkelstein DM, Ettinger DS,
Abeloff MD, Ruckdeschel JC, Eggleston JC. The clinical significance of
variant-morphology small-cell carcinoma of the lung. Journal of clinical
oncology. 1990;8(3):402-8.
19. Chute CG, Greenberg ER, Baron J, Korson R,
Baker J, Yates J. Presenting conditions of 1539 population‐based lung cancer
patients by cell type and stage in new hampshire and vermont. Cancer.
1985;56(8):2107-11.
20. Sahn SA. Malignancy metastatic to the pleura.
Clinics in chest medicine. 1998;19(2):351-61.
21. Decker DA, Dines DE, Payne WS, Bernatz PE,
Pairolero PC. The significance of a cytologically negative pleural effusion in
bronchogenic carcinoma. Chest. 1978;74(6):640-2.
22. Maskell N, Butland R. BTS guidelines for the
investigation of a unilateral pleural effusion in adults. Thorax. 2003;58(Suppl
2):ii8.
23. Rahman NM, Ali NJ, Brown G, Chapman SJ,
Davies RJ, Downer NJ, et al. Local anaesthetic thoracoscopy: British Thoracic
Society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii54-ii60.
24. Eren S, Karaman A, Okur A. The superior vena
cava syndrome caused by malignant disease: imaging with multi-detector row CT.
European journal of radiology. 2006;59(1):93-103.
25. Toloza EM, Harpole L, McCrory DC. Noninvasive
staging of non-small cell lung cancer: a review of the current evidence. Chest.
2003;123(1):137S-46S.
26. Schumacher T, Brink I, Mix M, Reinhardt M,
Herget G, Digel W, et al. FDG-PET imaging for the staging and follow-up of
small cell lung cancer. European journal of nuclear medicine. 2001;28:483-8.
27. Erasmus JJ, Patz Jr E, McAdams HP, Murray JG,
Herndon J, Coleman RE, et al. Evaluation of adrenal masses in patients with
bronchogenic carcinoma using 18F-fluorodeoxyglucose positron emission
tomography. AJR American journal of roentgenology. 1997;168(5):1357-60.
28. Quan AL, Videtic GM, Suh JH. Brain metastases
in small cell lung cancer. ONCOLOGY-WILLISTON PARK THEN HUNTINGTON THE MELVILLE
NEW YORK-. 2004;18:961-72.
29. Hiraki A, Ueoka H, Takata I, Gemba K, Bessho
A, Segawa Y, et al. Hypercalcemia–leukocytosis syndrome associated with lung
cancer. Lung Cancer. 2004;43(3):301-7.
30. Honnorat J, Antoine J-C. Paraneoplastic
neurological syndromes. Orphanet journal of rare diseases. 2007;2(1):1-8.
31. Hayes AR, Grossman AB. Distinguishing
Cushing's disease from the ectopic ACTH syndrome: Needles in a haystack or
hiding in plain sight? Journal of Neuroendocrinology. 2022;34(8):e13137.
32. Pignon J-P, Tribodet H, Scagliotti GV,
Douillard J-Y, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin
evaluation: a pooled analysis by the LACE Collaborative Group. Database of Abstracts of Reviews of Effects
(DARE): Quality-Assessed Reviews [Internet]: Centre for Reviews and
Dissemination (UK); 2008.
33. Pisters KM, Evans WK, Azzoli CG, Kris MG,
Smith CA, Desch CE, et al. Cancer Care Ontario and American Society of Clinical
Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA
resectable non–small-cell lung cancer guideline. Journal of clinical oncology.
2007;25(34):5506-18.
34. Soria J-C, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated
EGFR-mutated advanced non–small-cell lung cancer. New England journal of
medicine. 2018;378(2):113-25.
35. Wu Y-L, Tsuboi M, He J, John T, Grohe C,
Majem M, et al. Osimertinib in resected EGFR-mutated non–small-cell lung
cancer. New England journal of medicine. 2020;383(18):1711-23.
36. Felip E, Altorki N, Zhou C, Csőszi T,
Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant
chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010):
a randomised, multicentre, open-label, phase 3 trial. The Lancet.
2021;398(10308):1344-57.
37. Wu Y, Chen K, Xing W, Chen Q, Liu L, Zhang Q,
et al. 84P SHR-1316 vs placebo in combination with chemotherapy as
perioperative treatment in patients with resectable stage II-III NSCLC: A
randomized, double-blind, multicenter, phase Ib/III trial. Annals of Oncology.
2022;33:S72.
38. Siegel RL, Miller KD, Fuchs HE, Jemal A.
Cancer statistics, 2021. Ca Cancer J Clin. 2021;71(1):7-33.
39. Albain KS, Swann RS, Rusch VW, Turrisi AT,
Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without
surgical resection for stage III non-small-cell lung cancer: a phase III
randomised controlled trial. The Lancet. 2009;374(9687):379-86.
40. Aupérin A, Le Péchoux C, Rolland E, Curran
WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential
radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol.
2010;28(13):2181-90.
41. Vokes EE, Herndon JE, Kelley MJ, Cicchetti
MG, Ramnath N, Neill H, et al. Induction chemotherapy followed by
chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced
unresectable stage III non–small-cell lung cancer: Cancer and Leukemia Group B.
Journal of clinical oncology. 2007;25(13):1698-704.
42. Bradley JD, Hu C, Komaki RR, Masters GA,
Blumenschein GR, Schild SE, et al. Long-term results of NRG oncology RTOG 0617:
standard-versus high-dose chemoradiotherapy with or without cetuximab for
unresectable stage III non–small-cell lung cancer. Journal of Clinical
Oncology. 2020;38(7):706.
43. Faivre-Finn C, Vicente D, Kurata T, Planchard
D, Paz-Ares L, Vansteenkiste JF, et al. Four-year survival with durvalumab
after chemoradiotherapy in stage III NSCLC—an update from the PACIFIC trial.
Journal of Thoracic Oncology. 2021;16(5):860-7.
44. Antonia SJ, Villegas A, Daniel D, Vicente D,
Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III
non–small-cell lung cancer. New England Journal of Medicine.
2017;377(20):1919-29.
45. Rothschild SI, Zippelius A, Eboulet EI, Savic
Prince S, Betticher D, Bettini A, et al. SAKK 16/14: durvalumab in addition to
neoadjuvant chemotherapy in patients with stage IIIA (N2) non–small-cell lung
cancer—a multicenter single-arm phase II trial. Journal of clinical oncology.
2021;39(26):2872-80.
46. Temel JS, Greer JA, Muzikansky A, Gallagher
ER, Admane S, Jackson VA, et al. Early palliative care for patients with
metastatic non–small-cell lung cancer. New England Journal of Medicine.
2010;363(8):733-42.
47. Ettinger DS, Wood DE, Aisner DL, Akerley W,
Bauman JR, Bharat A, et al. Non-small cell lung cancer, Version 2.2021 featured
updates to the NCCN guidelines. JNCCN Journal of the National Comprehensive
Cancer Network. 2021;19(3):254-66.
48. Rizvi NA, Mazières J, Planchard D,
Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an
anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory
squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm
trial. The lancet oncology. 2015;16(3):257-65.
49. Borghaei H, Gettinger S, Vokes EE, Chow LQ,
Burgio MA, de Castro Carpeno J, et al. Five-year outcomes from the randomized,
phase III trials checkmate 017 and 057: nivolumab versus docetaxel in
previously treated non–small-cell lung cancer. Journal of Clinical Oncology.
2021;39(7):723.
50. Reck M, Rodríguez-Abreu D, Robinson AG, Hui
R, Csőszi T, Fülöp A, et al. Five-year outcomes with pembrolizumab versus
chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor
proportion score≥ 50%. Journal of Clinical Oncology. 2021;39(21):2339.
51. Herbst RS, Giaccone G, de Marinis F, Reinmuth
N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of
PD-L1–selected patients with NSCLC. New England Journal of Medicine.
2020;383(14):1328-39.
52. Sezer A, Kilickap S, Gümüş M, Bondarenko I,
Özgüroğlu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line
treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a
multicentre, open-label, global, phase 3, randomised, controlled trial. The
Lancet. 2021;397(10274):592-604.
53. Gadgeel S, Rodríguez-Abreu D, Speranza G,
Esteban E, Felip E, Dómine M, et al. Updated analysis from KEYNOTE-189:
pembrolizumab or placebo plus pemetrexed and platinum for previously untreated
metastatic nonsquamous non-small-cell lung cancer. Journal of clinical
oncology. 2020;38(14):1505-17.
54. Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in
metastatic non–small-cell lung cancer. New England journal of medicine.
2018;378(22):2078-92.
55. Riely GJ, Marks J, Pao W. KRAS mutations in
non–small cell lung cancer. Proceedings of the American Thoracic Society.
2009;6(2):201-5.
56. Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the
epidermal growth factor receptor underlying responsiveness of non–small-cell
lung cancer to gefitinib. New England Journal of Medicine. 2004;350(21):2129-39.
57. Paez JG, Janne PA, Lee JC, Tracy S, Greulich
H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical
response to gefitinib therapy. Science. 2004;304(5676):1497-500.
58. Pao W, Miller V, Zakowski M, Doherty J,
Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung
cancers from “never smokers” and are associated with sensitivity of tumors to
gefitinib and erlotinib. Proceedings of the National Academy of Sciences.
2004;101(36):13306-11.
59. Riely GJ, Ladanyi M. KRAS mutations: an old
oncogene becomes a new predictive biomarker. The Journal of Molecular
Diagnostics. 2008;10(6):493-5.
60. Riely GJ, Kris MG, Rosenbaum D, Marks J, Li
A, Chitale DA, et al. Frequency and distinctive spectrum of KRAS mutations in
never smokers with lung adenocarcinoma. Clinical cancer research.
2008;14(18):5731-4.
61. Brose MS, Volpe P, Feldman M, Kumar M, Rishi
I, Gerrero R, et al. BRAF and RAS mutations in human lung cancer and melanoma.
Cancer research. 2002;62(23):6997-7000.
62. Ostrem JM, Peters U, Sos ML, Wells JA, Shokat
KM. K-Ras (G12C) inhibitors allosterically control GTP affinity and effector
interactions. Nature. 2013;503(7477):548-51.
63. Soda M, Choi YL, Enomoto M, Takada S,
Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4–ALK
fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561-6.
64. Takeuchi K, Choi YL, Soda M, Inamura K,
Togashi Y, Hatano S, et al. Multiplex reverse transcription-PCR screening for
EML4-ALK fusion transcripts. Clinical Cancer Research. 2008;14(20):6618-24.
65. Koivunen JP, Mermel C, Zejnullahu K, Murphy
C, Lifshits E, Holmes AJ, et al. EML4-ALK fusion gene and efficacy of an ALK
kinase inhibitor in lung cancer. Clinical cancer research. 2008;14(13):4275-83.
66. Koh Y, Kim D-W, Kim TM, Lee S-H, Jeon YK,
Chung DH, et al. Clinicopathologic characteristics and outcomes of patients
with anaplastic lymphoma kinase-positive advanced pulmonary adenocarcinoma:
suggestion for an effective screening strategy for these tumors. Journal of
Thoracic Oncology. 2011;6(5):905-12.
67. Inamura K, Takeuchi K, Togashi Y, Nomura K,
Ninomiya H, Okui M, et al. EML4-ALK fusion is linked to histological
characteristics in a subset of lung cancers. Journal of thoracic oncology.
2008;3(1):13-7.
68. Inamura K, Takeuchi K, Togashi Y, Hatano S,
Ninomiya H, Motoi N, et al. EML4-ALK lung cancers are characterized by rare
other mutations, a TTF-1 cell lineage, an acinar histology, and young onset.
Modern Pathology. 2009;22(4):508-15.
69. Kwak EL, Bang Y-J, Camidge DR, Shaw AT,
Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in
non–small-cell lung cancer. New England Journal of Medicine.
2010;363(18):1693-703.
70. Togashi Y, Soda M, Sakata S, Sugawara E,
Hatano S, Asaka R, et al. KLC1-ALK: a novel fusion in lung cancer identified
using a formalin-fixed paraffin-embedded tissue only. PLoS One.
2012;7(2):e31323.
71. Solomon BJ, Mok T, Kim D-W, Wu Y-L, Nakagawa
K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive
lung cancer. New England Journal of Medicine. 2014;371(23):2167-77.
72. Daum G, Eisenmann-Tappe I, Fries H-W,
Troppmair J, Rapp UR. The ins and outs of Raf kinases. Trends in biochemical
sciences. 1994;19(11):474-80.
73. Cardarella S, Ogino A, Nishino M, Butaney M,
Shen J, Lydon C, et al. Clinical, pathologic, and biologic features associated
with BRAF mutations in non–small cell lung cancer. Clinical cancer research.
2013;19(16):4532-40.
74. Davies H, Bignell GR, Cox C, Stephens P,
Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature.
2002;417(6892):949-54.
75. Naoki K, Chen T-H, Richards WG, Sugarbaker
DJ, Meyerson M. Missense mutations of the BRAF gene in human lung
adenocarcinoma. Cancer research. 2002;62(23):7001-3.
76. Paik PK, Arcila ME, Fara M, Sima CS, Miller
VA, Kris MG, et al. Clinical characteristics of patients with lung
adenocarcinomas harboring BRAF mutations. Journal of clinical oncology.
2011;29(15):2046.
77. Pratilas CA, Hanrahan AJ, Halilovic E,
Persaud Y, Soh J, Chitale D, et al. Genetic predictors of MEK dependence in
non–small cell lung cancer. Cancer research. 2008;68(22):9375-83.