Exploring the platelet
and cancer cell interaction in metastasis targeting
Maria Riaz 1, Muhammad Zubair 1*, Muhammad
Kaleem Iqbal 2, Syed Muhammad Ahmad Bukhari 1, Hafiz
Muhammad Sultan 1
1 Institute of Biological Sciences,
Khwaja Fareed University of Engineering and Information Technology, Rahim Yar
Khan, Pakistan
2 Institute of Microbiology,
University of Agriculture Faisalabad, Pakistan
Corresponding Authors: Muhammad Zubair
* Email: zubairbiochem1334@gmail.com
Abstract
Platelets are small anucleated cell fragments
that ensure the stopping of bleeding. In blood metastasis of cancer, Platelets
are essential. One of the most important aspects of cancer metastasis is the
interaction between platelets and circulating tumor cells. Platelets are
involved in cancer spread and constitute a hazardous collation with the cancer
cells. There are various factors involved in hemostasis and thrombosis, which
can be activated by several cancer-related stimuli, including extracellular
matrix (ECM), adenosine diphosphate (ADP), and Toll-like receptors (TLRs).
Furthermore, it has been previously published that platelets build up inside
the main tumors, producing growth factors that encourage tumor growth and
angiogenesis. Additionally, tumor cells can interact with platelets through
aggregation, further protecting cancer cells. Platelets interact both
functionally and physically with different types of tumor cells via integrin
and other surface receptors. Platelet integrin’s primary function is to
maintain platelet adhesion and aggregation at vascular damage sites.
Pharmacological treatments that target integrin have been shown to effectively
inhibit experimental metastasis. This review paper summarized the recent
advances and progress of mechanisms in platelet activation and its interaction
with cancer cells in metastasis.
Keywords: Platelets, Cancer cells, Tumor, CTCs, Immune cells
Introduction
Platelets
are small fragments that are derived from megakaryocytes in bone marrow.
Circulating in the blood, platelets not only maintain hemostasis but also play
a vital role in cancer progression and metastasis (1) The
interaction between platelets and cancer cells promotes cancer metastasis (2). One aspect of
this interaction includes Circulating Tumor Cells (CTCs) (3). CTCs are
cancer cells that separate from the primary tumor and enter blood circulation (4). Platelets
bind to these CTCs and form a protective shield around them (5). This
protective shield protects the CTCs from immune cell detection and helps in
their dispersal to distant tissues. The interaction between CTCs and Cancer
metastasis is observed in different types of cancer including lung cancer,
colon cancer, and breast cancer. During cancer progression, a small number of
CTCs also invade nearby tissues by extravasation process thus contributing to
tumor angiogenesis (6).
Platelets
are disc-shaped blood cells, which consist of three types of granules,
Lysosomes, Dense granules, and Alpha granules. Alpha granules are present in
abundant and store various factors such as ADP/ATP, Fibrinogen, Extracellular
Matrix (ECM), and coagulation factors. Platelets release these growth factors
and molecules that stimulate angiogenesis, which promotes the formation of new
blood vessels around tumors and provides them with essential nutrients and
oxygen to grow and spread. Cancer cells also can activate platelets during
Cancer metastasis. Activated platelets and the release of various growth
factors enhance pro-thrombotic events. 25-30% of thrombotic events are
cancer-related (7). Cancer
patients encounter an increased occurrence of both arterial and deep vein
thrombosis. Activated platelets also release clotting factors that lead to the
formation of blood clots within the blood vessel during cancer (8).
Platelets
not only contribute to cancer metastasis but can also be used to target cancer
cells that are bound with the platelets, to treat cancer (9). Platelets
integrin's primary role is to maintain platelets aggregation and adhesion at
the vascular damage site. Targeting integrin has been shown to inhibit
experimental metastasis. In this review paper, we summarize the role of
platelets in different steps of cancer progression including cancer metastasis,
angiogenesis, and platelets-associated thrombosis development during cancer and
the development of platelets-based target therapies to treat cancer (10).
Interaction
between cancer cells and platelets
The
interaction between platelets and cancer cells initiates when a particular
molecule such as chemokines is released by cancer cells (11). These
molecules will function as a signal that will attract platelets to the tumor
microenvironment (12). A type of
chemical gradient is generated by these molecules that will direct platelets to
the tumor site (13). Interaction of cancer cells and platelets
also occurs by immediate receptor binding or by bridging of receptors by
Protein (14). For instance,
one platelet receptor engaged in Cancer progression is the CLEC-2 receptor that
in certain cancers binds with podoplanin. Podoplanin that are present on tumor cells interact with
the CLEC-2 receptors and leads to the activation of platelets that leads to
tumor growth and metastasis. However, platelets can also indirectly activated by releasing several proteins and growth factors
such as VEGF and PDGF that stimulates tumor growth and leads to cancer
progression. Different integrins
involved in cancer and platelet interaction includes αIIbβ3, αvβ3, α5β1, α6β1
and αvβ5 that bind specifically to their ligand fibrinogen, vitronectin,
fibronectin, laminin and vitronectin respectively. Although, the receptor
αIIbβ3 integrin plays a significant role in Cancer metastasis. It mediates the
interaction between Cancer Cells and platelets by adhesive proteins (such as
fibrinogen and von Willebrand Factor). The receptor αIIbβ3 goes through
structural changes after activation by interaction with platelet stimulants
such as ADP, collagen, thrombin, etc. (15). The receptor
αIIbβ3 shows an increased binding attraction to ligands (including fibrinogen
and WF) in its active form. By facilitating the cancer Cell and Platelets
aggregate's arrest in the endothelium, the receptor alphaIIbbeta3 also supports
the arrest of cancer cells in vessels (16). Platelets and
cancer cells interaction is a very diverse process that leads to cancer
metastasis.
Progression of cancer by platelets surface receptors
Platelets
surface receptors are a type of proteins that are present on the membrane of
platelets and promote the interaction between the platelets and cancer cells.
Various platelets surface receptors include GPIbα,
GPVI, P-selectin and GPIb-IX-V. Among them GPVI and GPIb-IX-V are platelets surface receptors that participate
in maintaining hemostasis, also imply the interaction among cancer cells and
platelets (17). They also
contribute a vital role in encouraging the Extravasation and the arrest of
Circulating Tumor Cells (CTCs) which are facilitated ultimately by the
progression of metastasis by adhesion proteins. GPVI is a vital receptor for
fibrin and collagen so; it facilitates the adhesion of platelets at the Injury
site. In vivo experiments performed on lung carcinoma and melanoma that lack
GPVI receptor in mice, show a 45% visible decrease in tumor (18). The
experiment performed on mice with cancer with defective GPIb-IX
shows a 14% decrease in metastatic foci. Although these receptors on platelets
are involved in cancer metastasis only, they are not involved in Primary tumor
growth. The activation of the platelets and adhesion of platelet-cancer cells
is also facilitated by the interplay among platelet and integrin. Integrin behave as a receptor that interplay with the ligands that
are present on the surface of both CTCs and platelets and thus contributes to
their adhesions. On the other hand, selectin can also contribute to the
adhesion between CTCs and platelets by promoting the interplay among CTCs and
platelets. When platelets are activated they express
P-selectin upon them, that binds to its ligand present on the CTCs and thus
contribute to the adhesion between CTCs and platelets. The range of interaction
among cancer Cells and Platelets does not depend upon a single
receptor-receptor pairing (19).
Platelets role in tumor angiogenesis
After
attaining a specific size, tumor cells have to initiate angiogenesis, in which
the tumor receives additional growth factors and nutrients that are necessary
for tumor cells to differentiate and spread into different parts of the body.
During Tumor angiogenesis, new blood vessels at the growing tumor site are
formed by the lining up of epithelial cells that are attracted by various
growth factors that are released in the tumor microenvironment by platelets and
lead to the formation of new capillaries and arteries (20). Platelet
α-granules are the main site for storing various factors that maintain
angiogenesis and hemostasis at the same time in the tumor microenvironment.
When platelets are activated, they release α-granules that contain various
growth factors that initiate angiogenesis, such as Vascular Endothelial Growth
Factor (VEGF) and Pro-angiogenic factors; epithelial cells and some
anti-angiogenic factors such as endostatin and thrombospondin-1 are also
released. The complex interaction among pro-angiogenic and anti-angiogenic
leads to the formation of pro-angiogenic and anti-angiogenic microenvironment
respectively. This interplay contributes to both the angiogenesis of tumor for
progression of cancer as well as understanding of these anti-angiogenic factors
can be used to inhibit cancer. Based on
stimuli that platelets receive from the external environment platelets can
particularly secrete various factors to initiate or prevent the development of
blood vessels in the developing tumor microenvironment (21). For example,
ADP-induced platelets can secrete VEGF but cannot release end statin;
meanwhile, thromboxane induces platelets to secrete endostatin rather than VEGF
(22). ADP secretes
VEGF in tumor microenvironment that is a pro-angiogenic factor and it is
released to promote tumor progression. Thromboxane releases endostatin in tumor
microenvironment that is an anti-angiogenic factor and it is released to limit
tumor vascularization.
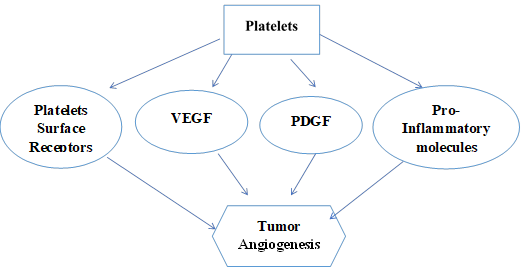
Figure 1. Demonstrates that various growth factors and receptors released by
platelets induce angiogenesis.
Platelets-induced release of Angiogenic factors
Platelets are also activated by various cancer
cells, these activation initiates the secretion of several substances such as
Angiogenic factors (23). These are the
substances that encourage the formation of new blood vessels (24). Currently, it
has also been developed that Stimulated Emission Depletion (STED) imaging can
also be used to demonstrate platelets-induced release of various growth factors
that initiate angiogenesis more accurately. Depending upon the external
stimuli, platelets can increase or suppress the angiogenesis of tumors by
particular secretion of pro or anti-angiogenic factors (25). Factors like
inflammation, hypoxia and shear stress act as external stimuli and contribute
to the release of pro-angiogenic and anti-angiogenic factors. For example, if
inflammatory signal is released by external environment, it will promote the
release of cytokines and growth factors which lead to the release of VEGF that
in result encourage angiogenesis. Although factors like nutrient availability
and pH can also contribute to the secretion of pro-angiogenic and
anti-angiogenic factors. Platelets selectively
intake and store VEGF in the α-granule that is released by a tumor in the tumor
microenvironment. However, tumors can also activate the secretion of VEGF by
platelet, thus maintaining the level of VEGF in the tumor microenvironment that
significantly initiates angiogenesis in the tumor microenvironment (26). Various other
angiogenic factors are also released by platelets including Fibroblast Growth
Factor (FGF) and Platelets Derived Growth Factors (PDGF) (27). FGF promotes
the migration of epithelial cells role and PDGF regulates the growth of muscle
cells both of them are essential for the formation of new blood vessels in
tumor angiogenesis. The Pro-angiogenic environment is established by these
angiogenic factors that will encourage cancer progression (28).
Platelets
encourage circulating tumor cells dispersal
Platelets
not only assist the growth of the primary tumor; however, but they also play an
important role in metastatic progression. They attach to the surface of
Circulating Tumor Cells and act as a
shield (29). This shield
of platelets serves as a camouflage for CTCs, due to which CTCs are very less
visible to immune cells. Platelets also make a cloak that surrounds CTCs,
deterring various immune cells from identifying them as a foreign particle.
This interplay prevents the CTCs from immune system detection and
recognition. Platelets aid these CTCs
when they encourage vasculature, which in turn assists the CTCs in the
bloodstream and dissemination of CTCs to different tissues. CTCs arrest could
be passive or active. During Passive arrest CTCs move in the bloodstream till
they attach to the platelets without any active contribution by CTCs. Passive arrest includes the blockage of CTCs
due to the formation of platelets, fibrinogen, and tumor cells in small blood
vessels (30). On the other
hand, active arrest includes the process in which platelets actively identify
and binds to CTCs and contributes to the development of aggregate that promotes
tumor cells survival. Active process refers to the transfer of tumor cells from
the primary tumor into the bloodstream.
Platelets
also act as a framework by covering the upper layer of circulating tumor cells
that aid CTCs to move freely in the bloodstream. Thus, platelets are core
regulators of tumor cells. When Platelets are activated by tumors they provide
various growth factors to the CTCs. Label et al. indicated that the secretion
of TGF-β (Transforming Growth Factor) by platelets and cancer cell-platelets
interaction initiates metastasis by stimulating various signaling pathways (31). When these
pathways are activated, they trigger Epithelial Mesenchyme Transition (EMT),
which is the process in which tumor cells having epithelial phenotype lose
their various features. EMT maintains the transfer of primary tumor cells into
the bloodstream, which leads to the dissemination of tumor cells to distant
tissues (32). Different
detection methods that are used to detect CTCs include PCR,
immunocytochemistry, flow cytometry and several approaches based on
microfluidics.
Platelets-induced
cancer cell reconfiguration
EMT
(Epithelial-Mesenchymal Transition) is a vital developmental program that also
takes place in cancer metastasis (33). Epithelial
cancer cells create a Key Mesenchymal cell layer via the Epithelial-Mesenchymal
Transition and alter their shape as they drop connection with the basement
membrane. The activity of Epithelial Mesenchymal Transition can be invertible
and epithelial cells can be converted into mesenchymal cells and vice versa.
Epithelial Mesenchymal Transition is also assisted by components of the
Extracellular Matrix, cells obtained from the microenvironment of tumor and
immune cells (34). Several
factors also participate in controlling Epithelial-Mesenchymal Transition
including Transcription Factors, Hepatocyte Growth Factors, and Transforming
Growth factors (TGF). TGF discharged by alpha granules of activated platelets
transforms Tumor cells into pro-metastatic EMT (35).
TGF
is activated by the interplay among platelet-cancer cells and platelets are
referred as a main source for TGF-β. TGF derived from platelets in cancer
cells, leading to the enhanced cancer metastasis and Epithelial-Mesenchymal
Transition phenotype in vivo. Altogether, these findings show a direct linkage
between EMT development and TGF released from platelets (36). However, TGF-β also activates Smad signaling
pathway that promotes EMT. Interaction of TGF-β with tumor cells receptor leads
to the activation of various Smad proteins that form complexes and move in to
the nucleus where they promote the expression of certain genes that leads to
the Epithelial Mesenchymal Transition. ECM components that are released by
tumor microenvironment or tumor are recommended for being involved in
Epithelial-Mesenchymal Transition. Cathepsin belongs to a group of protease
enzymes that are released by various tumor cells. Cathepsin is primarily
restrained in lysosomal vesicles and released as soluble enzymes that split ECM
components near cancer cells. Cathepsin also triggers platelet aggregation and
assists interplay of Epithelial-Mesenchymal Transition-Cancer Cells (5).
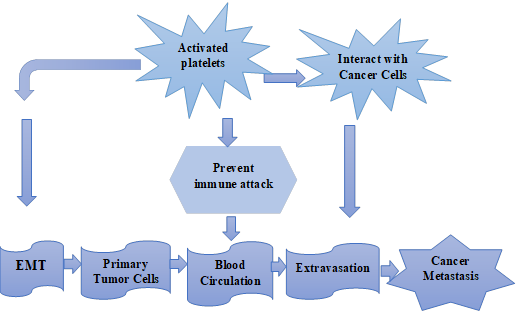
Figure
2. Schematic representation of activated platelets
interaction with cancer cells as well as initiate EMT and both of them induce
cancer metastasis.
Thrombosis
in cancer and tumor-induced platelet activation
Patients who suffer from cancer often face
blood-clotting problems in the various blood vessels that include both arteries
and veins. The development of thrombosis in cancer patients is another major
reason for mortality. Thrombosis elevates the possibilities of cancer
metastasis and progression that have been observed in lung and breast cancers
and it is associated with poor survival (37). There
are more chances of the development of thrombotic complications in cancer
patients in contrast to patients without cancer. Meanwhile, the accurate
procedure for the development of thrombosis in cancer is not completely
understood. However, more than one-fourth of the patients who suffer from
cancer have been diagnosed to have relatively high levels of platelets in their
blood (38).
Platelets that are activated by tumor cells can lead
to the development of thrombosis (39). Tumor
cell-induced platelets activation and aggregation (TCIPA) is detected in
fibro-blastoma. The main controller of this pathway is cancer cell Resident Podoplanin (PDPN). High expression of Podoplanin
increases the chances of thrombosis development during cancer. Podoplanin expression in epithelial cells can also increase
the risk of thrombotic complications. (40). When
platelets are indirectly activated by cancer cells they trigger the epithelial
cells to secrete various proteins and growth factors that provide an area for
platelets attachment and development of thrombosis (41). In
cancer patients development of Neutrophil extracellular trap (NET) is mostly identified that as contributing to
the elevated level of histone protein and other nucleosomes in the bloodstream (42). NET
leads to the development of tumor-induced thrombosis and dysfunction of various
organs (43). In
pancreatic cancer, NET is regarded as the main contributor to the development
of cancer. Elevated concentrations of TF were observed in these patients (44). These
findings show that platelets lead to thrombotic complications among cancer
patients (45).
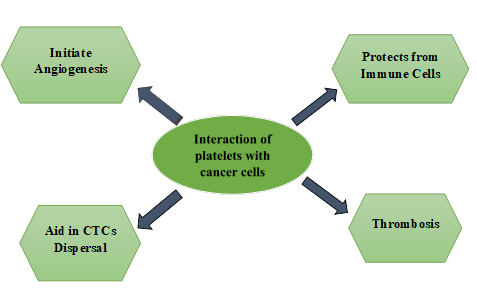
Figure 3. Diagrammatic representation
of the interaction between platelets and cancer cells that induce angiogenesis,
protection from immune cells, CTCs dispersal, and thrombosis in tumor
microenvironment.
Effect
of platelets on anti-tumor immunity
Platelets
perform very diverse roles in anti-tumor immunity activity (46). Among all
cancers, only a small number of cancer cells form metastatic foci. Natural
Killer (NK) cells are the immune cells that can remove cancer cells from blood
circulation. Platelets are the only blood cells that interact with the cancer
cells and form a protective shield around them that prevents them from immune
cell detection and recognition (47). Platelets
also protect tumor cells from anti-tumor immunity by the release of various
molecules that are immunosuppressive in their action (48). These
immunosuppressive molecules include Transforming Growth Factor Beta (TGF-β)
which can suppress the anti-tumor activity of various immune cells including NK
cells as well as T cells (49). TGF-β
inhibits NK cells and T cells activity by inhibiting their proliferation and
suppressing of cytotoxicity that leads to the immune tolerance and cancer
progression. Platelets also can suppress the activity of dendritic cells that
are crucial for regulating various immune responses against tumor cells (50). Platelets
suppress the activity of dendritic cells through various mechanisms such as by
direct physical interaction with dendritic cells that suppress their maturation
and by releasing various immunosuppressive molecules such as TGF-β and PGE2
that inhibit the function of dendritic cells and their capability to activate T
cells. Platelets not only play an important role in tumor angiogenesis but they
also maintain the integrity of the tumor, thus preventing hemorrhage of the
tumor (51). By regulating
the integrity of the tumor, platelets decrease the effect of the immune system
on the tumor. To survive in circulation Circulating Tumor Cells (CTCs) need to
protect themselves from immune system recognition and killing mechanism (52).
Platelets protect tumor cells from NK cells
Natural
Killer cells play an important role in Antitumor immunity activity (53). Platelets
that are activated along with fibrinogen shield the tumor cells and protect
them from Natural killer cells by the formation of a barrier that protects the
tumor cells from NK cells (54). This
protective shield makes it more difficult for NK cells to affect tumor cells.
Moreover, various immuno-suppressive molecules released by platelets also
diminish the activity of NK cells (55). A decrease in the level of Natural killer
cells will enhance metastasis of cancer. It has been shown that the platelets
induce metastasis of the tumor within 1 hour after the tumor has entered the
blood circulation meanwhile Natural killer cells employ their antitumor
immunity activity one and sixth hour after tumor extravasation. In comparison
to any other blood cells, platelets can keep a large quantity of Transforming
Growth Factor and secrete it into the microenvironment of the tumor during
metastasis and progression of cancer. It is demonstrated that the release of
this growth factor by platelets can lead to the down-regulation of Natural
Killer cells, thus inhibiting their antitumor immunity (56). As platelets
also promote tumor angiogenesis it is difficult for NK cells to eradicate tumor
cells (57).
Drugs
against tumor microenvironment
Different
types of receptors and cytokines present in the tumor microenvironment take
part in cancer metastasis (31). Many elements
that contribute to tumor metastasis assemble in the tumor microenvironment
making cancer treatment more difficult. The cancer resulting from
cancer-platelets interaction explains the fact that platelet is the main factor
that promote cancer by promoting angiogenesis, CTCs dispersal and protection
from immune system. Thus, targeting platelets will be the best strategy to
overcome the cancer progression resulting from cancer-platelet interaction. For
the molecules that are over-activated in cancer, various drugs have come into
being to target them (58). When it is
revealed that the platelets in cancer contribute to the suppression of the
immune system, an attempt to make a drug that will induce immune responses in
cancer was started (59). The best
strategy to inhibit cancer metastasis that is initiated by cancer-platelet
interaction is to use drugs that suppress the amount
of platelets in tumor site as well as use of chemotherapeutics that will use to
treat cancer. The microenvironment of the tumor helps us to understand how
tumors gain resistance against any antitumor drug. This concept is referred to
as “de novo mechanisms” that show how a change in the microenvironment
of a tumor can give tumor cells a new pathway to overcome the effect of
antitumor drugs (60).
Table 1. Some platelets targeting drugs that can
be used along chemotherapeutics in different types of cancers.
|
Cancer Cell type |
Platelets targeting Drugs |
Chemotherapeutics |
|
Human lungs
cells |
Aspirin |
Doxorubicin hydrochloride (Dox) |
|
Breast cancer
cells |
Trastuzumab |
Monomethyl auristatin E (MMAE) |
|
Human
leukemia |
Hydroxyurea |
Epidoxorubicin imaging Agent CY5 Carboxyfluorescein di-ester |
|
Human
lymphoma cells |
Rituximab |
Doxorubicin (Dox) |
|
Human colonic
carcinoma |
Oxaliplatin, Bevacizumab |
Tumor necrosis factor- Related apoptosis-inducing Ligand (TRAIL) |
|
Human triple
negative Breast cancer
cells |
Aspirin |
TRAIL |
Platelet is a
main target to overcome cancer metastasis
Platelets
and cancer cell interaction plays a major role in promoting CTCs dispersal to
distant tissues, angiogenesis, suppression of anti-tumor immunity activity, and
eventually cancer metastasis, so platelets are a main target to overcome cancer
metastasis (61). Metastasis of cancer is the major cause of
death in cancer patients. Clinical studies have shown that tumor cells that are
surrounded by platelets are less affected by chemotherapy. Furthermore,
platelets also encourage Epithelial-Mesenchymal Transition in tumor cells that
have chemoresistance (62). These studies
show that to overcome cancer metastasis effectively and completely targeting
platelets will be the best strategy. Inhibition of platelets in the clinical
model shows that it inhibits the metastasis of cancer. It is also demonstrated
that attachment of the platelets with the cancer cells, prevents them from
immune system recognition and attack, thus enhancing cancer metastasis (63). After
studying the vital role of platelets in cancer metastasis, it was demonstrated
that targeting platelets will be the best strategy to treat platelets-induced
cancer (64). Various drugs
that can suppress platelets can be used. These drugs can be transferred
directly to the tumor microenvironment. Although many drugs that can target
platelets also have tumor suppressive activity (65).
Platelet suppression by Aspirin and Integrin as a therapeutic target in cancer metastasis
Aspirin
is a common drug that is used to overcome fever and pain. Aspirin also can
suppress platelets, therefore it is used by patients with cardiac and
thrombotic complications (66).
Platelets-induced cancer metastasis can also be reduced by the use of aspirin.
It has also been shown in clinical experiments the growth and development of
cancer is reduced by aspirin. Aspirin function by suppressing the formation of
various chemicals such as prostaglandins that contributes to aggregation and
activation of platelets. By suppressing the amount of these chemicals, aspirin
assist in preventing platelets to adhere together and form clots. Tamoxifen is
another drug that is used in breast cancer as an antiestrogen (67). It is
demonstrated that tamoxifen suppresses metastasis of cancer that is induced by
platelets. Tamoxifen inhibit platelet activation by
altering the secretion of various angiogenic factors by platelets and by
suppressing the expression of various adhesion molecules on the surface of
platelets. Different types of integrins are expressed by platelets, such as
α6β1which facilitate the binding with collagen. Direct interplay among
platelets and collagen is regulated by GPVI and α6β1 integrin. Studies have shown
that the interplay among platelets and cancer cells that contribute to cancer
metastasis is terminated by blocking α6β1 integrin (68). The blocking
function of integrin with the help of antibodies will suppress the interaction
between cancer cells and platelets. Suppression of the function of integrin by
antibodies does not affect hemostasis and number of platelets in mice. This
antibody does not have any effect on cancer metastasis when introduced into
platelet α6β1 deficient mice. Integrin α6β1 is also found in endothelial cells
and pericytes, where they impart tumorigenic effect to the microenvironment of
a tumor. Inhibiting integrin α6β1 will suppress the different types of
integrin-facilitated cancer metastasis, thus inhibiting the function of this
integrin is one of the best strategies against cancer (69). There are
several integrin inhibitors involved in suppressing cancer metastasis in vivo
models such as Cilengitide, Volociximab and ATN-161. Cilengitide is an integrin inhibitor that targets αvβ5 and
αvβ3 and is a promising strategy in inhibiting clinical models by inhibiting
the ability of the primary tumor cells to spread at distant tissues.
Volociximab is a type of anti-angiogenic agent that inhibits the α5β1 integrin.
In clinical models it inhibits metastasis by preventing the development of
angiogenesis. ATN-161 is an integrin inhibitor that also inhibits α5β1. In
clinical models it inhibit metastasis by effecting
growth of tumors, angiogenesis and dispersal of CTCs to distant tissues.
Platelets-dependent
drug delivery to target primary tumor and platelets
carriers for cancer therapy
Platelets
also can take chemotherapeutics to tumor cells at two sites, in the
microenvironment of the tumor and blood circulation (70). For a long
period, platelets were used as blood clotting agents in blood circulations.
There are various ways to treat cancer but one of the best ways is to treat
cancer by using platelets as a carrier for the transfer of chemotherapeutics (71). Many factors
make the platelets a potential carrier to deliver the drug in the tumor
microenvironment (72). Its example
is Doxorubicin, which is filled with platelets by the using general incubation
method. This platelets-loaded Dox has been shown to inhibit the growth of
cancer in clinical models (73). The
platelets-based carrier has also been shown to inhibit tumors in mouse models (74). Entirely it
is demonstrated that the use of platelets as a carrier can expertly transfer
chemotherapeutics to the tumor microenvironment and inhibit platelets-induced
cancer (9). Additionally,
Yap et al. show that there is no side effect of using platelets-based carrier
on various functions of the organs. There is also research on using platelets
as a carries to transfer antibodies to be used as immunotherapeutic in which
antibodies are loaded into the membrane of platelets (75). In clinical
models, antibody-loaded platelets have been shown to inhibit the growth of
tumors (76).
Conclusion
Interaction
between Platelets and cancer cells plays a very important part in cancer
metastasis and progression. Platelets release various growth factors that help
CTCs to grow and spread into the different parts of the body and form
aggregates with them that protect them from the immune system. Cancer
cell-induced activation of platelets increases the risk of developing
thrombosis. Platelets also protect cancer cells from antitumor immunity
activity by forming a protective layer around tumor cells that acts as a shield
and prevents them from immune cell detection and killing mechanisms. Thus,
targeting interaction between platelets and cancer cells is the best strategy
to overcome cancer metastasis as well as cancer-induced thrombotic
complications. Treating strategies include specifically targeting primary
tumors, CTCs, and circulating malignancies. Among targeting strategies one of
the best strategies is to use platelets as a carrier to deliver
chemotherapeutics to tumor microenvironment. Meanwhile, delivery of the
chemotherapeutics using platelets gives us an excellent potential to treat
platelets-induced cancer but there are still many challenges that need to be
controlled.
Acknowledgment
We
would like to thank the Khawaja Fareed University of Engineering and
Information Technology Institute of Biological Sciences students who
participated in the data collection and were not listed in the author list.
Author
contribution
MR, MZ,
and SMAB design the study. MR, MZ, and HMS wrote
the first draft of the manuscript. MKI wrote a section of the
manuscript. All the authors contributed to the article and approved the
submitted version
Conflict
of interest
The
authors report no conflict of interest.
Funding
There
is no funding agency involved in this research.
References
1. Catani MV, et al. The
"Janus Face" of Platelets in Cancer. Int J Mol Sci. 2020;21(3).
2. Lucotti S, Muschel RJ. Platelets and metastasis: new implications of
an old interplay. Frontiers in oncology. 2020;10:1350.
3. Cendrowicz E, et
al. The role of macrophages in cancer development and therapy. Cancers.
2021;13(08):1946.
4. Lazar S, Goldfinger LE. Platelets and
extracellular vesicles and their cross talk with cancer. Blood, The Journal of
the American Society of Hematology. 2021;137(23):3192-200.
5. Zhou L, et al. The critical role of
platelet in cancer progression and metastasis. European Journal of Medical
Research. 2023;28(1):385.
6. Guo S, et al. The role of extracellular
vesicles in circulating tumor cell-mediated distant metastasis. Molecular
Cancer. 2023;22(1):193.
7. Mitrugno A, et
al. The role of coagulation and platelets in colon cancer-associated
thrombosis. American Journal of Physiology-Cell Physiology.
2019;316(2):C264-C73.
8. Kannan M, et al. Platelet activation
markers in evaluation of thrombotic risk factors in various clinical settings.
Blood reviews. 2019;37:100583.
9. Lu Y, et al. Platelet for drug delivery.
Current opinion in biotechnology. 2019;58:81-91.
10. Jiang Q, et al. Platelet membrane‐camouflaged
magnetic nanoparticles for ferroptosis‐enhanced cancer immunotherapy. Small.
2020;16(22):2001704.
11. Liao K, et al. The role of platelets in the
regulation of tumor growth and metastasis: the mechanisms and targeted therapy.
MedComm. 2023;4(5):e350.
12. Zhang X, et al. Research progress on the
interaction between oxidative stress and platelets: Another avenue for cancer?
Pharmacological Research. 2023:106777.
13. Oncul S, Cho MS.
Interactions between Platelets and Tumor Microenvironment Components in Ovarian
Cancer and Their Implications for Treatment and Clinical Outcomes. Cancers.
2023;15(4):1282.
14. Mege D, et al.,
editors. Involvement of platelets in cancers. Seminars in thrombosis and
hemostasis; 2019: Thieme Medical Publishers.
15. Amelirad A, et al.
Signaling pathways of receptors involved in platelet activation and shedding of
these receptors in stored platelets. Advanced Pharmaceutical Bulletin.
2019;9(1):38.
16. Mousavi SS, Razi S. Cell-in-cell structures
are involved in the competition between cells in breast cancer. arXiv preprint arXiv:211213271. 2021.
17. Mammadova-Bach E, et al. Platelet
glycoprotein VI promotes metastasis through interaction with cancer
cell–derived galectin-3. Blood, The Journal of the American Society of
Hematology. 2020;135(14):1146-60.
18. Rayes J, et al. Functional significance of
the platelet immune receptors GPVI and CLEC-2. The Journal of clinical
investigation. 2019;129(1):12-23.
19. Clemetson KJ, Clemetson JM. Platelet
receptors. Platelets: Elsevier; 2019. p.
169-92.
20. Jiang X, et al. The role of microenvironment
in tumor angiogenesis. Journal of Experimental & Clinical Cancer Research.
2020;39(1):1-19.
21. Sabrkhany S, et al.
Platelets as messengers of early-stage cancer. Cancer and Metastasis Reviews. 2021;40:563-73.
22. Lacroix R, et al., editors. Involvement of
platelets in cancers. Seminars in thrombosis and hemostasis; 2019: Thieme
Medical Publishers 333 Seventh Avenue, New York, NY 10001, USA.
23. Segal BH, et al. Neutrophil interactions with
T cells, platelets, endothelial cells, and of course tumor cells. Immunological
Reviews. 2023;314(1):13-35.
24. Xu H, et al. Rapid formation of ultrahigh
strength vascular graft: Prolonging clotting time micro-dimension hollow
vessels with interpenetrating polymer networks. Composites Part B: Engineering.
2023;250:110456.
25. Neophytou CM, et al. The role of tumor
microenvironment in cancer metastasis: Molecular mechanisms and therapeutic
opportunities. Cancers. 2021;13(9):2053.
26. Teleanu RI, et al.
Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. Journal
of clinical medicine. 2019;9(1):84.
27. Sedighi M, et al. An overview of angiogenesis
and chemical and physiological angiogenic factors: short review. Journal of
Chemical Health Risks. 2023.
28. Liu Z-L, et al. Angiogenic signaling pathways
and anti-angiogenic therapy for cancer. Signal Transduction and Targeted
Therapy. 2023;8(1):198.
29. Anvari S, et al. Interactions of platelets
with circulating tumor cells contribute to cancer metastasis. Scientific
Reports. 2021;11(1):15477.
30. Vismara M. Blood platelets and cancer: the
involvement of platelet-derived extracellular vesicles in tumour
progression. 2021.
31. Żmigrodzka M, et
al. Platelets extracellular vesicles as regulators of cancer progression—An
updated perspective. International Journal of Molecular Sciences.
2020;21(15):5195.
32. Xin Y, et al. Fluid shear stress induces EMT
of circulating tumor cells via JNK signaling in favor of their survival during
hematogenous dissemination. International journal of molecular sciences.
2020;21(21):8115.
33. Gundamaraju R, et al. Autophagy and EMT in
cancer and metastasis: Who controls whom? Biochimica
et Biophysica Acta (BBA)-Molecular Basis of Disease.
2022;1868(9):166431.
34. Aggarwal V, et al. Interplay between tumor
microenvironment and partial EMT as the driver of tumor progression. IScience. 2021;24(2).
35. Wang X, et al. Platelets involved tumor cell
EMT during circulation: communications and interventions. Cell Communication
and Signaling. 2022;20(1):1-12.
36. Guo Y, et al. Platelets promote invasion and
induce epithelial to mesenchymal transition in ovarian cancer cells by TGF-β
signaling pathway. Gynecologic oncology. 2019;153(3):639-50.
37. Khorana AA, et al. Cancer-associated venous
thromboembolism. Nature Reviews Disease Primers. 2022;8(1):11.
38. Suzuki-Inoue K. Platelets and
cancer-associated thrombosis: focusing on the platelet activation receptor
CLEC-2 and podoplanin. Hematology 2014, the American
Society of Hematology Education Program Book. 2019;2019(1):175-81.
39. Palacios-Acedo A-L, et al. Platelet and
cancer-cell interactions modulate cancer-associated thrombosis risk in
different cancer types. Cancers. 2022;14(3):730.
40. Shi Q, et al. The role of tumor-platelet
interplay and micro tumor thrombi during hematogenous tumor metastasis.
Cellular Oncology. 2023:1-12.
41. Palacios-Acedo AL, et al. Platelets,
thrombo-inflammation, and cancer: collaborating with the enemy. Frontiers in
immunology. 2019;10:1805.
42. Kaltenmeier C, et
al. Neutrophil extracellular traps (Nets) in cancer metastasis. Cancers.
2021;13(23):6131.
43. De Meo ML, Spicer
JD, editors. The role of neutrophil extracellular traps in cancer progression
and metastasis. Seminars in immunology; 2021: Elsevier.
44. Dymicka-Piekarska
V, et al. Inflammatory cell-associated tumors. Not only macrophages (TAMs),
fibroblasts (TAFs) and neutrophils (TANs) can infiltrate the tumor
microenvironment. The unique role of tumor associated platelets (TAPs). Cancer
Immunology, Immunotherapy. 2021;70(6):1497-510.
45. Alekseeva LA, et al. Targeting circulating
SINEs and LINEs with DNase I provides metastases inhibition in experimental
tumor models. Molecular Therapy-Nucleic Acids. 2020;20:50-61.
46. Guo J, et al. Exploitation of platelets for
antitumor drug delivery and modulation of the tumor immune microenvironment.
Acta Materia Medica. 2023;2(2):172-90.
47. Schmied L, et al. Platelet-mediated
protection of cancer cells from immune surveillance–possible implications for
cancer immunotherapy. Frontiers in immunology. 2021;12:640578.
48. Stoiber D, Assinger A. Platelet-leukocyte
interplay in cancer development and progression. Cells. 2020;9(4):855.
49. Larson C, et al. TGF-beta: a master immune
regulator. Expert opinion on therapeutic targets. 2020;24(5):427-38.
50. Xue VW, et al. Transforming growth factor-β:
a multifunctional regulator of cancer immunity. Cancers. 2020;12(11):3099.
51. van den Bulk J, et al. Therapeutic targeting
of TGF-β in cancer: hacking a master switch of immune suppression. Clinical
Science. 2021;135(1):35-52.
52. Castro-Giner F, Aceto N. Tracking cancer
progression: From circulating tumor cells to metastasis. Genome Medicine.
2020;12(1):1-12.
53. Nicholson SE, et al. Natural killer cells and
anti-tumor immunity. Molecular Immunology. 2019;110:40-7.
54. Clar KL, et al. Inhibition of NK reactivity
against solid tumors by platelet-derived RANKL. Cancers. 2019;11(3):277.
55. Wolf NK, et al. Roles of natural killer cells
in immunity to cancer, and applications to immunotherapy. Nature Reviews
Immunology. 2023;23(2):90-105.
56. Li Z, et al. The role of platelets in tumor
growth, metastasis, and immune evasion.
Platelets: Elsevier; 2019. p. 547-61.
57. Daher M, Rezvani K. Outlook for new CAR-based
therapies with a focus on CAR NK cells: what lies beyond CAR-engineered T cells
in the race against cancer. Cancer discovery. 2021;11(1):45-58.
58. Bejarano L, et al. Therapeutic targeting of
the tumor microenvironment. Cancer discovery. 2021;11(4):933-59.
59. García-Aranda M, Redondo M. Immunotherapy: a
challenge of breast cancer treatment. Cancers. 2019;11(12):1822.
60. Pai Bellare G, et al. Targeting autophagy
reverses de novo resistance in homologous recombination repair proficient
breast cancers to PARP inhibition. British Journal of Cancer.
2021;124(7):1260-74.
61. Lai V, et al. Drug delivery strategies in
maximizing anti-angiogenesis and anti-tumor immunity. Advanced Drug Delivery
Reviews. 2021;179:113920.
62. Dudás J, et al. Epithelial to mesenchymal
transition: a mechanism that fuels cancer radio/chemoresistance. Cells.
2020;9(2):428.
63. Braun A, et al. Platelet-cancer interplay:
molecular mechanisms and new therapeutic avenues. Frontiers in oncology. 2021;11:665534.
64. Cacic D, et al. Platelets for advanced drug
delivery in cancer. Expert Opinion on Drug Delivery. 2023(just-accepted).
65. Yu L, et al. Bidirectional interaction
between cancer cells and platelets provides potential strategies for cancer
therapies. Frontiers in Oncology. 2021;11:764119.
66. Tao DL, et al. Aspirin and antiplatelet
treatments in cancer. Blood, The Journal of the American Society of Hematology.
2021;137(23):3201-11.
67. Pather K, et al.
Breast cancer cell-induced platelet activation is compounded by tamoxifen and
anastrozole in vitro. Thrombosis Research. 2019;177:51-8.
68. Aksorn N, Chanvorachote P. Integrin as a molecular target for
anti-cancer approaches in lung cancer. Anticancer research. 2019;39(2):541-8.
69. Li M, et al. Integrins as attractive targets
for cancer therapeutics. Acta Pharmaceutica Sinica B.
2021;11(9):2726-37.
70. Dovizio M, et al.
Multifaceted functions of platelets in cancer: from tumorigenesis to liquid
biopsy tool and drug delivery system. International Journal of Molecular
Sciences. 2020;21(24):9585.
71. Li Q-R, et al. Platelets are highly efficient
and efficacious carriers for tumor-targeted nano-drug delivery. Drug Delivery.
2022;29(1):937-49.
72. Huong PT, et al. The role of platelets in the
tumor-microenvironment and the drug resistance of cancer cells. Cancers.
2019;11(2):240.
73. Ren D, et al. Mesoporous Doxorubicin-Loaded
Polydopamine Nanoparticles Coated with a Platelet Membrane Suppress Tumor
Growth in a Murine Model of Human Breast Cancer. ACS Applied Bio Materials.
2021;5(1):123-33.
74. Wang L, et al. Emerging roles of platelets in
cancer biology and their potential as therapeutic targets. Frontiers in
Oncology. 2022;12:939089.
75. Wang Y, et al. Engineered platelets:
Advocates for tumor immunotherapy. Nano Today. 2021;40:101281.
76. Xu M, et al. Thalidomide prevents
antibody-mediated immune thrombocytopenia in mice. Thrombosis Research. 2019;183:69-75.