A
histopathological study on breast carcinoma with special reference to cyclin-D1
and estrogen receptor
Payel Hazari 1, Monoj Kumar Deka 1, Bandana
Kanoo 1
1 Department of Pathology, Silchar Medical College and Hospital,
India
Corresponding Authors: Payel Hazari
* Email: hazari.payel2@gmail.com
Abstract
Introduction: Breast cancer is the most frequent cause of
cancer-related death in women in developing nations. Breast cancer diagnoses
have increased as a result of rising awareness among women. The expression of
Estrogen receptors (ER) plays a crucial role in determining the responsiveness
to specific treatments. Cyclin D1 being a marker for cell proliferation was
used in this study. The primary objectives of the current investigation were to
investigate the expression of Cyclin-D1 and Estrogen receptor (ER) in breast carcinoma
and to establish a relationship between the expression patterns of Cyclin-D1
and ER with the histopathological features of the tumor in breast carcinoma.
Materials and methods: The study was conducted in the Department of Pathology, Silchar
Medical College and Hospital, Silchar, India, from June 2021 to May 2022. A
total of 59 cases of primary breast carcinoma MRM(Modified radical
mastectomy) specimens were included in
the study.
Results: The mean age of the patients was 52.12 ± 12.47 years, and the majority
of the patients were in the post-menopausal phase. Lymph node metastasis was
observed in 47.5% of the cases, and the majority of the cases were in grade II.
The study demonstrated a trend towards increased Cyclin-D1 and ER-positive with
aging. Cyclin-D1 positivity decreases and Cyclin-D1 negativity increases as the
tumor growth increases. The study showed a statistically significant
association (P=0.001) between ER and Cyclin-D1. The majority of post-menopausal
patients had ER-positive.
Conclusion: The present study provides the incidence of different parameters
associated with breast carcinoma and their statistical correlation with
CyclinD1 and ER that will provide improved and crucial treatment guidance.
Keywords: Breast carcinoma, Histopathological grades, Lymph node metastasis,
Estrogen receptor (ER), Cyclin-D1, Menopausal status
Introduction
Breast carcinoma accounts for 1 in 4 cancer diagnoses
among women worldwide. Breast cancer, which accounts for an anticipated
2,261,419 cases (11.7% of all cancer sites) each year (2020) and 684,996
fatalities (6% of all cancer-related deaths), is the most common worldwide
(2020). Breast cancer is the most frequent cause of cancer-related death in
women in developing nations. In India, 178 361 (26.3%) new cases were found
among the female population in 2020 (1). Breast cancer diagnoses have increased
as a result of rising awareness among women.
Breast cancer has a high rate of survival when
detected early and when there is access to effective therapy. Unfortunately,
50–80% of these illnesses are found at an advanced stage in the majority of
low- and middle-income countries (2). A more sensitive assessment of a palpable
breast lump has recently been employed with the help of the Triple Test
approach, which consists of a clinical examination, mammography, and fine
needle aspiration cytology (3). Early diagnoses of aggressive tumors (ER-ve,
PR-ve, HER2/neu +ve, or triple-negative tumors) result from increased awareness
campaigns (4, 5, 6).
As per the latest treatment guidelines for breast
cancer (7), the expression of estrogen receptors (ER) plays a crucial role in
determining the responsiveness to specific treatments. The ER expressions are
critical in determining how well hormonal therapy will function (8).
Histopathologists commonly assess tumor proliferation
activity, which provides data on the clinical behavior, diagnosis, and
treatment of tumors (9). Cyclins bind to and activate Cyclin-Dependent Kinases
(CDK), regulating the rate at which cells transition between different cell
cycle phases. In this study, cyclin D1 was used as a marker for cell
proliferation.
Cyclin D1 activates steroid hormone receptor-mediated
transcription in the absence of estrogen hormone and enhances transcription in
its presence. The anti-estrogens did not inhibit the activation of estrogen
receptors by Cyclin D1.There is an increase in binding of the receptor to
estrogen response element sequence that upregulates ER-mediated transcription
owing to the direct binding of Cyclin-D1 to the hormone binding domain of ER.
These results highlight a unique role for Cyclin D1 as a CDK-independent matter
of the ER (10).
This study aims to investigate various parameters like
age, laterality, menopausal status, tumour size, lymph node and the expression
of ER and Cyclin-D1 in breast cancer and to establish a relationship between
the expression patterns of Cyclin-D1 and ER with the histopathological features
of the tumor in breast carcinoma. This will provide improved and crucial
treatment guidance for breast cancer patients.
Materials and Methods
The present study was undertaken to study the
clinic-pathological findings in breast carcinoma and to assess the expression
of Cyclin-D1 and ER in them.
Place of study
The present study was undertaken in the Department of
Pathology, Silchar Medical College and Hospital, Silchar. The study was
approved by the Institute’s Ethics Committee (No. SMC/15,222) dated 20/10/2022. According
to the Helsinki Declaration’s ethical guidelines, the study is compliant.
Study period
1 year: From June 2021 to May 2022.
Type of Study
Hospital-based
prospective cross-sectional study.
Source of data and sample size
59 cases of primary breast carcinoma MRM specimens
submitted to the Department of Pathology, Silchar Medical College and Hospital,
Silchar, for histopathological
examination (Figure 1). Immunohistochemistry with CyclinD1 and ER antibody was
done on these specimens as per IHC protocol.
A

B

Figure 1. Gross pictures an
MRM specimen (A is anterior view; B is posterior view).
Inclusion criteria
In the study, patients with invasive duct carcinoma,
no special type (IDC, NST) as histopathological diagnoses were included.
Exclusion criteria
- All metastatic carcinoma of breasts.
- Male breast carcinomas
Parameters studies
I. Detailed
clinical history is taken and all routine investigations are done after
obtaining consent from the patients.
II. Hospital
records of the patients.
III.
Microscopic examination of the tissues.
IV. Immunohistochemistry
on paraffin embedded tissue of histopathologically diagnosed cases.
The current study was conducted prospectively at a
hospital in Silchar, India, in the Department of Pathology during a year, from
2021 to 2022. 59 biopsy/resection specimens for primary breast carcinoma were
submitted in total. All regular investigations are carried out after obtaining
the patients' agreement and a thorough clinical history is gathered. These
specimens were first stained with H&E before being subjected to
immunohistochemistry using CyclinD1 and ER antibodies by the IHC methodology.
Preparation of slides: Paraffin
sections were cut and mounted on saline coated slides. The slides were heated
at 65![]() to remove the
paraffin and then immerse in xylene. After rehydration of the tissues, the
slides were cleaned with distilled water. Subsequently, the slides were washed
with Tris buffer and submerged in a 3% peroxide solution for three minutes to
remove endogenous peroxidase activity.
to remove the
paraffin and then immerse in xylene. After rehydration of the tissues, the
slides were cleaned with distilled water. Subsequently, the slides were washed
with Tris buffer and submerged in a 3% peroxide solution for three minutes to
remove endogenous peroxidase activity.
Antigen detection and antigen retrieval: Heat retrieval was performed using a decloaking chamber with citrate
buffer at 95![]() for 40 minutes. The slides were then transferred to
Tris-Saline buffer to cool to room temperature. To prevent non-specific
immunostaining , the tissue sections were treated with 1% mouse serum. Primary
antibodies, including Rabbit monoclonal antibody QR022 for CyclinD1 and Rabbit
monoclonal antibody QR013 for ER were applied to the sections approximately one
hour before removal.
for 40 minutes. The slides were then transferred to
Tris-Saline buffer to cool to room temperature. To prevent non-specific
immunostaining , the tissue sections were treated with 1% mouse serum. Primary
antibodies, including Rabbit monoclonal antibody QR022 for CyclinD1 and Rabbit
monoclonal antibody QR013 for ER were applied to the sections approximately one
hour before removal.
Secondary detection of the primary antibody: After 10 minutes of incubation with biotinylated mouse anti-species
antibody, sections were washed in Tris buffer. The slides were then treated
with a solution of chromogen 3,3’- diaminobenzidine (DAB) at a concentration of
1mg/mL in Tris buffer containing 0.016% fresh H2O2. Tap water was used to clean
the DAB from the slides.
Counterstaining: Slides were immersed in a solution of
hematoxylin diluted 1:1 with distilled water for counterstaining. After
counterstaining, the slides were cleaned in distilled water and dehydrated by
dipping them in ethanol. Then a coverslip was used to view and report after
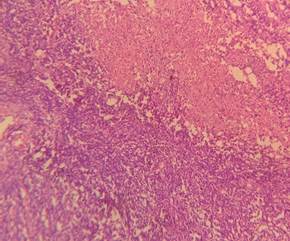
cleaning in xylene (Figure 2).
A
B
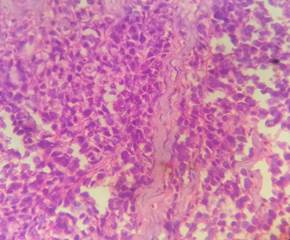
Figure 2. H&E pictures
of IDC, NST (A: 10X and B: 40X).
Reporting of CyclinD1 immuno-histochemical study
A semi-quantitative scoring is used by the Allred
score method for the nuclear staining (11) as
-
0: negative (no staining of any nuclei even at high magnification)
-
1: weak (only visible at high magnification)
-
2:moderate (readily visible at low magnification)
-
3: strong (strikingly positive even at low power magnification).
Additionally noted was the percentage of tumor nuclei
that stained positively as:
0- none, 1- <1/100, 2- 1/100
to 1/10, 3- 1/10 to 1/3, 4- 1/3 to 2/3 and
5- >2/3.
After that, the intensity scores and proportion were
combined to get a final score that varied from 0 to 8 (11).
Tumors were then categorized as:
-
Negative/weak expression (total scores 0–2)
-
Intermediate expression (total scores 3–5)
-
Strong expression (total scores 6–8)
In this study, Intermediate and Strong positives were
considered together as positive.
Reporting of ER immunohistochemical study
Strong brown to black nuclear staining was considered
when assessing immune positivity for ER. Positive nuclei were expressed as the
percentage of total nuclei counted.
Criteria for evaluating ER (12)
-
Negative for ER: If, 1% or 0% of tumor cell nuclei are immunoreactive.
-
ER Low Positive: If 1%-10% of tumor cell nuclei are immunoreactive.
-
Positive for ER: 1%-100% of tumor nuclei are immunoreactive.
Statistical analysis
IBM SPSS
software version 21.0 was used for data analysis. Qualitative data was
presented as frequency and percentage, while quantitative data was presented as
mean (![]() SD). The chi-square test was used to identify
significant associations. A p-value of <0.05 was regarded as
statistically significant.
SD). The chi-square test was used to identify
significant associations. A p-value of <0.05 was regarded as
statistically significant.
Results
In our study, various clinicopathological parameters
are analyzed and are presented as under.
The mean age of the patients having breast carcinoma
was 52.12
± 12.47 years and the majority of the patients belonged to 41 to 50 years of
age (32.2%). This was followed by 28.8% and 18.6% cases belonging to the age
range of 51 to 60 years and ![]() 40 years of age
respectively (Table 1).
40 years of age
respectively (Table 1).
Table 1. Distribution according to age.
|
Age (in years) |
Frequency (n = 59) |
Percentage (%) |
|
≤40 |
11 |
18.6 |
|
41 – 50 |
19 |
32.2 |
|
51 – 60 |
17 |
28.8 |
|
61 – 70 |
07 |
11.9 |
|
>70 |
05 |
8.5 |
|
Mean |
52.12 ± 12.47 |
|
In the present study, right-side predominance was
observed for breast carcinoma. 52.5% of patients had carcinoma breast on the
right breast while 47.5 % were over
the left breast. (Table 2).
Table 2. Distribution
according to laterality of breast carcinoma.
|
Laterality |
Frequency (n = 59) |
Percentage (%) |
|
Left |
28 |
47.5 |
|
Right |
31 |
52.5 |
In our study, 33.9% of patients were in a
pre-menopausal state and a majority of the cases 66.1% were in the
post-menopausal phase. We considered menopause where no menstruation was
reported over the last 12 months. (Table 3).
Table 3. Distribution
according to menopausal status.
|
Menopausal status |
Frequency (n=59) |
Percentage (%) |
|
Pre-menopausal |
20 |
33.9 |
|
Post
–menopausal |
39 |
66.1 |
Most commonly affected (40.7%) cases of breast
carcinoma patients had tumour of size 2-5cm. This was followed by ≤2cm tumour
size in 30.5% and >5cm in 28.8% cases respectively (Table 4).
Table 4. Distribution
according to tumour size.
|
Size of tumour |
Frequency (n = 59) |
Percentage (%) |
|
≤2cm |
18 |
30.5 |
|
2-5cm |
24 |
40.7 |
|
>5cm |
17 |
28.8 |
In
our study, lymph node metastasis was observed in 47.5% of cases whereas, in
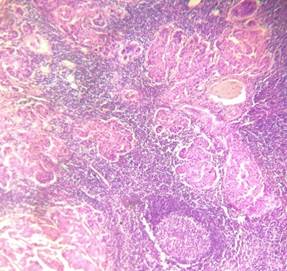
52.5% of cases, no lymph node metastasis was documented (Table 5) (Figure 3).
Table 5. Distribution according to lymph node metastasis of Breast carcinoma.
|
Metastasis |
Frequency (n = 59) |
Percentage (%) |
|
Present |
28 |
47.5 |
|
Absent |
31 |
52.5 |
A
B
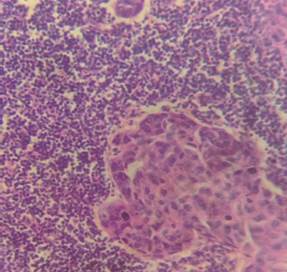
Figure 3. H&E picture
of metastatic lymph node (A: 10X and B: 40X).
The Nottingham
(Elston-Ellis) modification of the Scarff-Bloom-Richardson grading system also
called as the Nottingham Grading System is applied for the above grading. The
majority of cases 61% were found to be in grade II, this was followed by 30.5%
and 8.5% in grade III and I respectively (Table 6).
Table 6. Distribution
according to the histological grades of the tumours.
|
Tumour grade |
Frequency (n=59) |
(%) |
|
I |
5 |
8.5 |
|
II |
36 |
61 |
|
III |
18 |
30.5 |
Tumour
cells with >10% nuclear staining were regarded as positive and <10% or
weak staining as negative. In this study, we found 36 out of 59 cases (61%)
showed CyclinD1 positive expression whereas 23 cases (39%) cases had negative
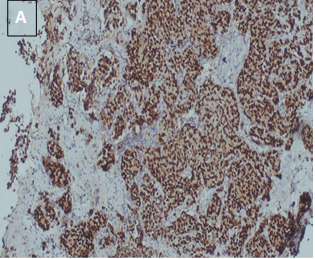
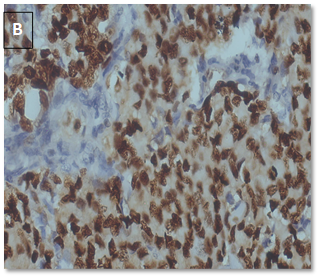
CyclinD1 expression (Table 7) (Figure 4).
Table 7. Distribution
according to the expression of CyclinD1 in breast carcinoma.
|
CyclinD1 expression |
Frequency
(n = 59) |
Percentage (%) |
|
Positive |
36 |
61 |
|
Negative |
23 |
39 |
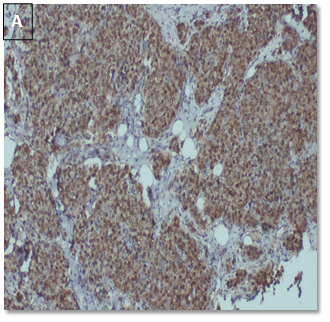
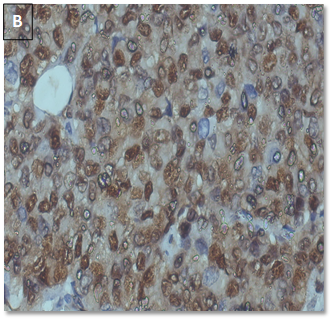
Figure 4. CyclinD1 positive
in a case of IDC, NST (A: 10X, B: 40X).
For
calculating the statistical significance, we grouped 59 cases into 2 categories
based on their age as ≤40 years and >40 years age. The majority of patients,
48 out of 59(81.4%) are over 40 years of age and 11 (18.6%) are ≤40 years of
age. 94.4% of patients >40 years show positive CyclinD1 expression and 60.9%
of cases are negative for CyclinD1. However, 5.6% of cases of ≤40 years of age show positive CyclinD1
expression. The test of
significance (chi-square test) showed a statistically significant association
between age and CyclinD1 expression in the present study (χ2 = 8.334,
P-value=0.0039) (Table 8).
Table 8. Association
between age and CyclinD1 expression.
|
Age (in years) |
Total cases (n=59) |
CyclinD1 positive (n=36) |
CyclinD1 negative (n=23) |
P-value |
|
≤40 |
11 (18.6%) |
2(5.6%) |
9(39.1%) |
0.0039 |
|
41-50 |
19 (32.3%) |
14 (38.9%) |
5 (21.7%) |
|
|
51-60 |
17 (28.8%) |
10(27.8%) |
7 (30.4%) |
|
|
61-70 |
07 (11.9%) |
06(16.6%) |
01(4.4%) |
|
|
>70 |
05 (8.5%) |
04(11.1%) |
01 (4.4%) |
For calculating the p-value,
we grouped the tumour size into 2 categories: ≤2 (18 cases) and >2cm (41 cases). CyclinD1 expression was seen in
47.2% of tumours with ≤2cm tumour size and 52.8%tumours with size >2cm. The
difference was statistically significant (χ2 = 10.230, P-value=0.014). Also, the majority (65.2%) of CyclinD1 negative
tumours have a size >5cm, followed by 2-5cm and ≤2cm with 30.4% and
4.4% respectively. This shows that with
an increase in tumour size there is an increase in Cyclin-D1 negativity (Table
9).
Table 9. Association between tumour size and CyclinD1 expression.
|
Tumour size (in
cm) |
Total cases
(n=59) |
CyclinD1
positive (n= 36) |
CyclinD1
negative (n=23) |
P-value |
|
≤2 |
18 (30.5%) |
17 (47.2%) |
01 (4.4%) |
0.014 |
|
2-5 |
24 (40.7%) |
17 (47.2%) |
07 (30.4%) |
|
|
>5 |
17 (28.8%) |
02 (5.6%) |
15 (65.2%) |
In this study, from 36
overexpressed Cyclin-D1 cases, the majority of cases 80% are in grade I. This
is followed by grade II and grade III with 69.4% and 38.9% cases respectively.
For calculating the statistical significance (p-value) of this correlation, we
grouped grade I and II as intermediate grade and grade III alone as high grade.
This correlation was found to be statistically significant (p-value=0.0435).
This implies Cyclin-D1 nuclear positivity is associated with lower tumour
histological grade (Table 10).
In this study, from 40
ER-positive cases, the majority of cases 80% are in grade I. This is followed
by grade II and grade III with 69.4% and 61.1% cases. For calculating the
statistical significance (p-value) of this correlation, we grouped grade I and II
together as intermediate grade and grade III alone as high grade. This
correlation was found to be statistically insignificant (p-value>0.05)
(Table 10).