Inflammatory
cytokines as diagnostic biomarkers in esophagus cancer
Agheel Tabar Molla Hassan 1 *
1 Department of Cell and Molecular
Biology, Babol Branch, Islamic Azad University, Babol, Iran
Corresponding Authors: Agheel Tabar Molla Hassan
* Email: doctoragheel@yahoo.com
Abstract
One of the most important types of proteins related to inflammation is
cytokines which are considered potential biomarkers of esophageal cancer. In
this way, these biomarkers, in conjunction with imaging techniques, may prove
practical in the diagnosis and monitoring of therapy for various malignancies,
such as esophageal cancer. Remarkably, in this article, the
importance of cytokines is demonstrated to declare its practical applications on
the dysregulation of cytokines in esophagus cancer and their clinical and
pathological implications in diagnosis and also therapy. It has been confirmed
that twenty-two cytokines exhibit abnormal levels in patients with esophageal
cancer. Correspondingly, MIF is related to the regulation of growth processes,
and IL-1β, IL-6, and IL-8 are related directly to regulation in the transcription
process. IL-1β and IL-6 stimulate the production of proinflammatory cytokines. Additional research is crucial to determine the
biological significance of cytokines in esophageal
cancer, including their potential for early diagnosis, pre- and post-operative
prognosis, and monitoring the response to chemotherapy and radiotherapy in
cancer patients..
Keywords: Inflammatory cytokines, diagnostic biomarkers, esophagus cancer,
Mechanism
Introduction
Esophageal
cancer is one of the most common cancers worldwide and the eighth leading cause
of cancer (1). Esophageal
squamous cell carcinoma (ESCC) is the most common histological type of
esophageal cancer (1, 2). This cancer
is usually found in the advanced stages of the disease and local or distant
metastases appear (2, 3).
For
this reason, the prognosis and survival prognosis of patients with ESCC is
poor. In ESCC, the lack of serosa and the abundance of submucosal lymphatic
structures favor disease spread during the disease (3). For that
reason, most ESCC patients have micrometastases that
are not visible at the tumor site at diagnosis. Tumor resection is an important
method of treatment for ESCC. Therefore, radiotherapy and chemotherapy are
complementary treatment methods (4).
Advances
in clinical observations involving imaging techniques such as endoscopy,
computed tomography (CT), magnetic resonance imaging, and positron emission
tomography are useful for detecting esophageal dysplasia or neoplasms. These
methods are very accurate in determining how to treat cancer. However, in some
cases, the cancer is said to have advanced at the time of surgery, and in most
cases treatment is straightforward. The two biggest risk factors for ESCC are
smoking and alcohol consumption (5).
These
include a variety of chemical carcinogens that stimulate inflammatory
responses, induce oxidative stress parameters, disrupt genetics, alter enzyme
activity, and induce angiogenesis (5). In recent
years, it has been shown that the oncogenic transformation of cells is
indicated at the molecular level, among others, by changes in the expression of
proteins (6, 7).
This
protein may be a marker for the early progression of esophageal cancer. New
biomarkers close to imaging techniques may help in the diagnosis and treatment
of patients with ESCC. It is important to identify biomarkers using simple and
non-invasive methods. Several studies were performed using enzyme-linked
immunosorbent assay (ELISA), Western blot (WB), immunohistochemistry (IHC),
proteomic s, and airway mass and found a reduction in serum and tumor tissue
protein levels. in ESCC patients. Studies on the relationships between protein
alterations and clinical and pathological parameters may reveal the role of
these molecules as ESCC biomarkers (7-9).
Biological
Role of Cytokines and Growth Factors
Cytokines
belong to a group of soluble proteins of low molecular weight. They act as
mediators between cells, establish cell growth processes, and participate in
differentiation, migration, and apoptosis (10, 11). Various types
of specialized cells of the innate and adaptive immune system secrete it.
Cytokines affect various cellular functions through specific receptors. It
plays an important role in immunity, inflammation, repair, tissue homeostasis,
and hematopoiesis (11). Cytokines are
characterized by pleiotropy, reduction, synergism, and antagonism (12, 13).
The
group of cytokines currently includes many elements with different origins and
functions. Therefore, it isn't easy to classify these peptides. In composition,
it includes interleukins (IL), interferons (IFN), chemokines (IL-8), and growth
factors such as transforming growth factor beta (TGF-β), vascular endothelial
growth factor (VEGF), and epidermal growth factor.
In
practice, cytokines are both proinflammatory (eg
IL-1, IL-6, IL-8, IL-18, IFN-γ, TNF-α, TNF-β and FasL)
and anti-inflammatory factors (eg IL- 4, IL-10, and
TGF-β). (11, 13, 14). Many
inflammatory cytokines have been implicated in various mechanisms leading to
cancer (14, 15). It is well
known that the process of malignant transformation is closely related to
abnormal responses in cytokine expression (13, 15). Cytokines
also play an important role in stimulating tumorigenic angiogenesis and
inducing metastasis (13).
Cytokines
in SCC of Esophagus
The
tumor microenvironment contains not only cancer cells but also fibroblasts,
endothelial cells, immune cells, and cytokines, which play an important role in
the regulation of this type of cell communication (14, 16-18). Under certain
conditions, in the early stages of cancer development, endogenous cytokines can
stimulate host immune responses against tumor cells. However, current data
suggest that many of these factors contribute to poor prognosis and contribute
to tumor growth, progression, metastasis, and clinical resistance (11, 12). For these
reasons, changes in the ESCC microenvironment may have important implications
for cancer development. Like other malignant tumors, esophageal cancer cells
secrete several disease-causing factors to suppress the host's defenses (8, 9).
The
cytokine network of ESCC is rich in proinflammatory cytokines, growth factors,
and chemokines. In this study, I demonstrated that 22 cytokines are associated
with clinical and pathological symptoms and survival rates of ESCC. The
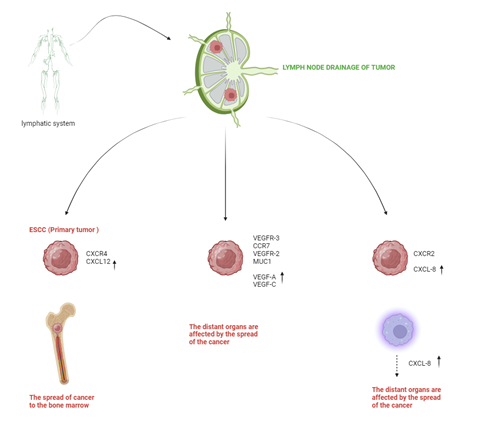
main cytokines associated with ESCC are VEGF-A, VEGF-C, VEGF-D, bFGF, HGF, MIF, TGF-β, IL-6, IL-8 and FasL,
and midkine, IL-18, PDGF-BB, CTGF and CXCL12 (19, 20).
High
expression of VEGF family members, including HGF and bFGF,
was observed ESCC tumor tissues, suggesting their potential role in the
processes of tumor growth, angiogenesis, and metastasis (21, 22). In addition,
VEGF-A, C, D, IL-8, IL-6, IL-18, TGF-β, HGF, FasL,
PDGF-BB, and midkine members influence tumor
progression, lymph node metastasis, and distant metastasis. parameters (20, 23). The functions
of ESCC-derived cytokines are shown in Table 1. MIF is associated with
the regulation of growth processes, and IL-1β, IL-6, and IL-8 are associated
according to the transfer law. IL-1β and IL-6 are stimulators of
proinflammatory cytokine production. FasL induces
apoptosis of activated lymphocytes in the host's immune system, thereby causing
cancer (24, 25).
Transforming
growth factor beta (TGF-β) plays a dual role in tumor development. In the early
stages of cancer, these cytokines act as tumor suppressors, but later they
promote tumor invasion by stimulating extracellular matrix formation, tumor
growth, angiogenesis, and abolishing military blockade (26).
Although
most cytokines are known to play a role in cancer growth and metastasis, other
cytokines such as IL-2, IL-12, IL-23, IL-27, and IFN-γ exert anticancer
responses through various molecular mechanisms (27).
Table
1. Cellular
role of ESCC-associated cytokines.
|
Function
|
Cytokine
|
|
Immune suppression
|
TGF-β
|
|
Growth regulation
|
MIF
|
|
Transcription regulation
|
·
IL-1β
·
IL-6
·
IL-8
|
|
·
Inflammatory
·
cytokines
secretion
|
·
IL-1β
·
IL-6
|
|
Apoptosis negative regulation
|
FasL
|
|
·
Host immune
stimulation
·
(Th1
response)
|
·
IL-2
·
IFN-γ
·
IL-12
·
IL-18
|
|
Angiogenesis stimulation
|
·
VEGF-A
·
VEGF-C
·
VEGF-D
·
IL-8
·
HGF
·
bFGF
·
PDGF-BB MIF
|
|
Metastasis induction
|
·
VEGF-A
·
VEGF-C
·
VEGF-D
·
bFGF
·
HGF
·
midkine
|
Cytokines
as a marker for the presence of ESCC
Histologic
changes in the development of ESCC include mild to malignant epithelial
dysplasia, localized carcinoma, and invasive carcinoma (2). The
pathogenesis of esophageal cancer is still unclear. Molecular studies have
shown that genetic changes, as well as alcohol consumption and smoking, are
responsible for pathological changes in the squamous epithelium of the
esophagus (2, 28, 29). Early
detection of this type of cancer is the most effective way to treat patients
with ESCC.
Therefore,
there is a need to find changes in cytokine levels related to tumorigenesis. In
this review, 22 cytokines showed different levels in ESCC patients. Among these
peptides, 20 showed higher levels, and only IL-2 and IFN-γ showed lower levels
were reported (25, 30). IHC studies
showed higher expression of selected cytokines in esophageal cancer tissue
compared to normal tissue (20).
This
suggests that the regulation of these peptides is involved in growth and
progression. on tumor expression of VEGF-A, VEGF-C, TGF-β, IL-1β, IL-6, HGF,
CTGF, CXCL12, FAS-L, a-FGF, bFGF, IGFBP7, IGF-II, midkine and MIF . Research has
shown the relationship between cytokine production and cancer (21, 31-34).
However,
the biological function of these cytokines in tumor cells and the tumor
microenvironment is different. On the other hand, the expression of cytokines
in tumor cells promotes tumor growth and stimulates oncogenic transformation;
on the other hand, the production of these peptides in the immune cells of the
tumor microenvironment can also contribute to the antitumor immune response (35). Most studies
analyzed cytokine expression in esophageal tissue samples using the IHC method.
However, these researchers examined the expression of cytokines in tumor
tissues in both early and advanced TNM stages of cancer. There are no studies
analyzing cytokine expression only in early stage ESCC. The lack of this type
of study is due to the high invasiveness of ESCC and therefore to the small
group of patients with early-stage cancer. Serum cytokines are positively
correlated with tumor stage, angiogenesis, and metastasis. The biological
significance of circulating cytokines in esophageal cancer is currently
unknown. One of the hypotheses indicates that high serum levels of some
cytokines can be associated with apoptosis induced by activated lymphocytes,
which facilitates tumor cell progression and metastasis. This deactivation of
host immune surveillance may be important for circulating cancer cells in the
blood and lymph nodes (25).
Several
ELISA studies, including ours, have shown significantly higher levels of
VEGF-A, VEGF-C, VEGF-D, TGF-β, IL-6, IL-8, IL-12, IL-18, PDGF- BB, HGF, FasL, MIF and midkine levels in
the serum of ESCC patients (23, 33, 36-38). The
relationship between serum concentrations of VEGF-A, VEGF-C, VEGF-D, IL-12,
IL-18, PDGF-BB, HGF, FasL, and midkine
and cancer stage was shown (25, 39-41). Analysis of
IFN-γ and IL-2 showed that the serum levels of these factors were significantly
reduced in patients with ESCC (20).
Both
cytokines are important inducers of Th1-related inflammatory responses and
inhibit cancer development (13, 20). Based on the
IHC and ELISA studies, we think that VEGF-A, VEGF-C, and HGF are useful
biomarkers for the clinical diagnosis of the presence of ESCC, but they may not
be useful for the early detection of esophageal cancer (30, 33, 35,
42-44). Serum levels
of VEGF-A, VEGF-C, VEGF-D, and TGF-β were found to be increased in ESCC
patients and significantly decreased after surgical treatment (23). Serum
analysis of these cytokines may help monitor treatment efficacy in patients
with ESCC (Figure 1).
miRNAs
MiRNAs
are highly conserved, non-coding single-stranded small RNA molecules encoded by
endogenous genes and about 20–24 nucleotides in length (56). They can
participate in the regulation of several biological functions, including cell
differentiation, apoptosis, proliferation, and metabolism by regulating the
expression of target genes (57). In 2002,
Calin et al found that miRNAs are downregulated in chronic B lymphocytic
leukemia, the first report of a link between miRNAs and tumors. Currently,
miRNAs are thought to mediate post-transcriptional regulation of gene
expression mainly through both target mRNA degradation and inhibition of
protein translation (58). More and more
studies have shown that different miRNAs play different roles in promoting
cancer or tumor suppression, and those aberrantly expressed miRNAs can
unbalance the expression of oncogenic or suppressive genes in the body,
ultimately leading to the formation of tumors (59). MiRNAs not
only have abnormal expression in tumor tissues but also have specific
expression in patient serum. Recent studies have shown that tumor-derived
miRNAs are resistant to endogenous ribonuclease activity, so they can be
present in a stable form in human serum. In addition, serum miRNA expression
levels are reproducible and consistent between individuals, making them ideal
candidates for blood diagnostic screening. Because Zhang et al (60). serum miRNA
levels in ESCC patients were first reported in 2010, several studies have
investigated the differential expression of circulating miRNAs and their
potential applications in ESCC (61). Thus, miRNA
markers found in circulation may play a role in enabling the early diagnosis of
ESCC. Until now, more and more studies have confirmed that c-miRNA can be used
as a new serum molecular marker for the early diagnosis of ESCC. Most studies
focused on candidate miRNAs selected from previous ESCC tissue analysis, while
other investigators used high-throughput technology to analyze miRNAs in
discovery sample datasets and then performed qRT-PCR
on an independent validation dataset to determine tissue diagnostic value.
candidate miRNAs (62). From 33
manuscripts, a total of 43 different types of miRNAs were investigated in the
serum of ESCC patients. In these studies, the sensitivity, specificity, and AUC
of miRNAs for the diagnosis of ESCC were 55.3-96.9%, 47.4-100%, and
0.590-0.951, respectively. Among the most studied individual miRNAs in ESCC are
known miRNAs such as miR-21, miR-223, miR-375, miR-25, and miR-100 (63).It analyzed
the diagnostic value of miR-21 and found that it has good sensitivity and
specificity for ESCC, which are 71.0% and 96.9%, respectively. However, the
number of ESCC patients included in the study was small, and the lack of miR-21
validation studies limits clinical expansion. This study reports the analysis
of a test and validation panel of serum miRNAs that may be potential diagnostic
biomarkers for ESCC. The combination of a test cohort and a validation cohort
significantly improved the reliability of diagnostic accuracy compared to many
previous studies without a validation cohort. For example, the serum level of
miR-1322 gave an area under the curve of the receiver operating characteristic
(ROC) of 0.847 (95% CI: 0.795-0.890), which was used to discriminate between
ESCC and healthy controls in the experimental group. Similar results were
obtained in the valid group (area under the ROC curve: 0.845; 95% CI:
0.780-0.897) (64). They showed
that the seven miRNA profile can be used as a
biomarker of ESCC and, more importantly, that it has the potential to predict
early ESCC. The study found that a panel of seven miRNAs was a more sensitive
marker for ESCC than the conventional biomarker carcinoembryonic antigen.
created a diagnostic model of serum miRNAs in 566 ESCC patients and 4965
control patients, the largest study to date to design ESCC diagnostic models.
This article used two independent cohorts to study the diagnostic model
consisting of miR-8073, miR-6820-5p, miR-6794-5p, miR-3196, miR-744-5p, and
miR-6799-5p. The sensitivities/specificities were 100%/98.0% and 96.0%/98.0%,
respectively, with similar diagnostic values in early ESCC (64). In addition,
Li et al (61) reviewed 18
publications and investigated 39 different types of miRNAs in EC patients. The
authors report relatively high sensitivity and specificity of combined and
individual miRNA markers, indicating some value in diagnostic application. The
results showed that single miRNAs did not show statistically significantly
better accuracy than multiple miRNA panels, which is contrary to some previous
studies (61). Since only
two studies reported in this article compared multi-miRNA panels, this finding
may not be sufficient to support such a conclusion. Many studies have shown
that circulating miRNAs in serum have potential clinical use as early tumor
diagnostic markers, but more clinical data and mechanistic studies are needed. Our
current knowledge about miRNAs can be boiled down to this, first, the
transcription of a single miRNA may require the simultaneous regulation of
multiple miRNAs. On the other hand, a single miRNA can be involved in the
simultaneous regulation of the expression of several mRNAs (65). Second,
processing and detection methods for serum circulating miRNA have yet to be
standardized, and the selection of internal parameters requires further
verification and standardization. Finally, most studies on serum circulating
miRNAs in early tumor diagnosis include small-sample, single-center studies,
while large-sample, multicenter, prospective clinical trials are needed.
Long
non-coding RNAs
Long
non-coding RNAs (lncRNAs) are non-coding RNAs longer than 200 bases that lack
an open reading frame and therefore lack protein-coding capacity (66). LncRNAs
regulate gene expression at different levels. LncRNAs regulate gene expression
and act differently than miRNAs, which not only affect protein
post-translational regulation but also act in multiple ways that affect gene
transcriptional activity and protein degradation (67, 68). A large body
of evidence indicates that lncRNAs have cancer-promoting or cancer-preventing
effects by influencing tumor cell proliferation, invasion, metastasis,
differentiation, apoptosis, and genomic stability (69).
HOX-transcribed RNA (HOTAIR) is the first long noncoding RNA with transregulatory effects in primary and metastatic breast
cancer. In addition, some studies found that HOTAIR is also highly expressed in
ESCC tissues, and the expression level is inversely correlated with
differentiation grade and positively correlated with TNM stage (70). Previous
studies on lncRNAs have mainly focused on cancer tissues. (71). In recent
years, researchers have studied the expression levels of lncRNAs in the serum
or plasma of cancer patients, and many studies have shown that lncRNAs can also
exist in other body fluids, including serum, plasma, and other body fluids. Not
sure. Furthermore, a study by Arita et al confirmed that lncRNAs remain in
circulating blood under certain conditions (72). Recently,
several laboratories have proposed different serum or plasma lncRNAs that can
be used for early diagnosis and monitoring of the severity of ESCC. Wang et al (73) qRT-PCR analysis
revealed elevated levels of HOTAIR in the serum of ESCC patients. However,
further investigation is needed to determine the sensitivity and specificity of
this finding, as initial data suggests a specificity of 56.0%. Furthermore,
serum levels of HOTAIR decrease after ESCC surgery. These results suggest that
serum lncRNA-HOTAIR may be a molecular marker for ESCC. Several studies have
shown that lncRNAs tested individually or in combination have similar or
superior diagnostic performance to traditional cancer biomarkers. The levels of
three lncRNAs, POU3F3, HNF1A-AS1, and SPRY4-IT1, in the plasma of patients with
ESCC, were significantly higher than those of normal controls, in plasma POU3F3
show a very strong correlation (area under the curve 0.842), sensitivity 72.8%,
specificity 89.4%) (74). In 147 ESCC
and 123 healthy controls, plasma POU3F3 and squamous cell carcinoma antigen
(SCCA) were found to have good detection and improved diagnostic performance
(area under the curve 0.926, sensitivity 85.7%, specificity 81.4% ). 80.8% of patients with early ESCC were detected,
suggesting that the combination of POU3F3 and SCCA may be useful for early
detection of ESCC (74). Circulating
lncRNAs are thought to be stable in blood because of encapsulation in microvesicles or exosomes (72). A better
understanding of the transport of lncRNAs within and between cells and the
basic biology of cell-derived lipid vesicles may help to develop biomarkers for
the detection of human diseases in circulating lncRNAs. In addition, the
detection of biomolecular markers in peripheral blood has the advantage of a
simple and minimally invasive surgery. Therefore, finding new lncRNAs as
diagnostic molecular markers in blood circulation is expected to be a hot
scientific topic in the field of biomarker research. To introduce circulating
lncRNAs into clinical practice, further research and improvements on the
standardization of sample preparation methods, the control of endogenous
lncRNAs in body fluids, and the combination of extraction methods should be performed.
Criteria for evaluating lncRNA quality and reliability of qPCR results should
be accurate and reliable while minimizing selectivity (71). Most of the
current studies were designed with small samples and have no real clinical
application. Therefore, it is necessary to expand the sample size and combine
multicenter clinical validation studies to develop lncRNA detection kits to
detect markers in blood to improve the efficiency of early detection and
subsequent investigation of the function of lncRNAs in tumors.
Conclusion
The
article showed that certain cytokines play a role in the aggressive nature of
ESCC and are associated with primary tumor progression, lymphatic and distant
metastases, and patient outcomes. VEGF family members appear to play an
important role as early markers of ESCC. In addition, HGF and bFGF may serve as specific prognostic markers for ESCC.
Changes in levels of angiogenic cytokines and growth factors, such as VEGF-A,
VEGF-C, TGF-β, and HGF, and microvascular assessment can be used to indicate lymphangiogenesis and distant metastasis in patients with
ESCC. Cytokines play an important role in tumor growth, angiogenesis, and
metastasis, but their role in ESCC is not fully understood. Further studies are
needed to confirm the biological significance of cytokines in ESCC and their
utility for early diagnosis, staging, and response monitoring of chemotherapy
and radiotherapy cancer patients. Among all proteins related to inflammation,
cytokines play an important role in cancer development and progression and may
be implicated as possible biological markers of esophageal cancer.
Author
contribution
ATMH designed the statistical analysis and wrote the paper.
Conflict
of interest
There
is no conflict of interest.
Funding
There
is no funding agency involved in this research.
References
1. Maron SB, Catenacci DV.
Novel targeted therapies for esophagogastric cancer. Surg Oncol Clin N Am.
2017;26(2):293-312.
2. Schizas D, et al. Adenosquamous
carcinoma of the esophagus: a literature review. J Transl
Int Med. 2018;6(2):70-3.
3. Kumagai Y, et al. Angiogenesis in
superficial esophageal squamous cell carcinoma: assessment of microvessel density based on immunostaining for CD34 and
CD105. Jpn J Clin Oncol. 2014;44(6):526-33.
4. Dermanis AA, et
al. The Evolution of Neo-Adjuvant Therapy in the Treatment of Oesophageal and Gastro-Oesophageal
Junction Adenocarcinomas. Cancers. 2023;15(19):4741.
5. Shah MA, et al. Improving outcomes in
patients with oesophageal cancer. Nat Rev Clin Oncol.
2023;20(6):390-407.
6. Huang F-L, Yu S-J. Esophageal cancer: risk
factors, genetic association, and treatment. Asian J Surg. 2018;41(3):210-5.
7. Kumagai Y, et al. Tumor-associated
macrophages and angiogenesis in early stage esophageal
squamous cell carcinoma. Esophagus. 2016;13:245-53.
8. Wang M, et al. Tissue protein biomarker
candidates to predict progression of esophageal squamous cell carcinoma and
precancerous lesions. Ann N Y Acad Sci.
2018;1434(1):59-69.
9. Yang X, et al. Targeted proteomics-derived
biomarker profile develops a multi-protein classifier in liquid biopsies for
early detection of esophageal squamous cell carcinoma from a population-based
case-control study. Biomark Res. 2021;9:1-12.
10. Rybkina V, et al.
The Role of Cytokines in the Pathogenesis of Malignant Neoplasms. Cell and
Tissue Biology. 2023;17(6):608-18.
11. Laha D, et al. The role of tumor necrosis
factor in manipulating the immunological response of tumor microenvironment.
Front Immunol. 2021;12:656908.
12. Ralli M, et al. The role of cytokines in head
and neck squamous cell carcinoma: A review. Clin Ter. 2020;171(3):268-74.
13. Kondoh N,
Mizuno-Kamiya M. The role of immune modulatory cytokines in the tumor
microenvironments of head and neck squamous cell carcinomas. Cancers.
2022;14(12):2884.
14. Serefoglou Z, et
al. Genetic association of cytokine DNA polymorphisms with head and neck
cancer. Oral Oncol. 2008;44(12):1093-9.
15. Piotrowski I, et al. Interplay between
inflammation and cancer. Rep Pract Oncol Radiother. 2020;25(3):422-7.
16. Saito S, et al. Stromal fibroblasts are
predictors of disease-related mortality in esophageal squamous cell carcinoma.
Oncol Rep. 2014;32(1):348-54.
17. Whiteside T. The tumor microenvironment and
its role in promoting tumor growth. Oncogene. 2008;27(45):5904-12.
18. Eiró N, Vizoso FJ. Inflammation and cancer.
World J Gastrointest Surg. 2012;4(3):62.
19. Kleespies A, et al. Clinical significance of
VEGF-A,-C and-D expression in esophageal malignancies.
Onkologie. 2005;28(5):281-8.
20. Delko T, et al. Cytokine response in the
pleural fluid and blood in minimally invasive and open esophagectomy. World J
Surg. 2019;43:2631-9.
21. Hosono M, et al. CXCL8 derived from
tumor-associated macrophages and esophageal squamous cell carcinomas
contributes to tumor progression by promoting migration and invasion of cancer
cells. Oncotarget. 2017;8(62):106071.
22. Wu G-Z, et al. Clinicopathological
significance of Fas and Fas
ligand expressions in esophageal cancer. Am J Cancer Res. 2015;5(9):2865.
23. Xu X, et al. The predicting role of serum
tumor-specific growth factor for prognosis of esophageal squamous cell
carcinoma. BMC cancer. 2023;23(1):1067.
24. Kase S, et al. Expression of Fas and Fas ligand in esophageal
tissue mucosa and carcinomas. Int J Oncol. 2002;20(2):291-7.
25. Kozlowski M, et al. Serum soluble Fas ligand (sFasL) in patients
with primary squamous cell carcinoma of the esophagus. Folia Histochem Cytobiol.
2007;45(3):199-204.
26. Ishiguro H, et al. Nuclear
expression of TCF4/TCF7L2 is correlated with poor prognosis in patients with
esophageal squamous cell carcinoma. Cell Mol Biol Lett. 2016;21:1-8.
27. Yan J, et al. Interleukin (IL)-12 and IL-23
and their conflicting roles in cancer. Cold Spring Harb Perspect
Biol. 2018;10(7):a028530.
28. Lam AK. Introduction: Esophageal squamous
cell carcinoma—current status and future advances. Methods Mol Biol. 2020:1-6.
29. Szumiło J.
Epidemiology and risk factors of the esophageal squamous cell carcinoma. Pol Merkur Lekarski.
2009;26(151):82-5.
30. Shih C-H, et al. Vascular endothelial growth
factor expression predicts outcome and lymph node metastasis in squamous cell
carcinoma of the esophagus. Clin Cancer Res. 2000;6(3):1161-8.
31. Koide N, et al. Histochemical study of
vascular endothelial growth factor in squamous cell carcinoma of the esophagus.
Hepatogastroenterology. 1999;46(26):952-8.
32. Ren Y-J, Zhang Q-Y. Expression of midkine and its clinical significance in esophageal
squamous cell carcinoma. World J Gastroenterol. 2006;12(13).
33. Shiratori F, et al. The effectiveness of
serum midkine in detecting esophageal squamous cell
carcinoma. Esophagus. 2019;16:246-51.
34. Li Y, et al. Integrated Bioinformatics
Analysis for Identifying the Significant Genes as Poor Prognostic Markers in
Gastric Adenocarcinoma. J Oncol. 2022;2022.
35. Tanaka T, et al. Vascular endothelial growth
factor C (VEGF-C) in esophageal cancer correlates with lymph node metastasis
and poor patient prognosis. J Exp Clin Cancer Res. 2010;29:1-7.
36. Xia H, et al. Overexpression of VEGF-C
correlates with a poor prognosis in esophageal cancer patients. Cancer Biomark. 2016;17(2):165-70.
37. Kozlowski M, et al. Serum vascular
endothelial growth factors C and D in patients with oesophageal
cancer. Eur J Cardiothorac
Surg. 2010;38(3):260-7.
38. Pastrez PRA, et al.
Interleukin-8 and interleukin-6 are biomarkers of poor prognosis in esophageal
squamous cell carcinoma. Cancers. 2023;15(7):1997.
39. Tullavardhana T, et
al. Vascular endothelial growth factor-C expression as a biomarker of poor
prognosis in esophageal squamous cell carcinoma: a meta-analysis. 2015. p.
110-4.
40. Fukai Y, et al. Reduced expression of
transforming growth factor‐β receptors is an unfavorable prognostic factor in
human esophageal squamous cell carcinoma. Int J Cancer. 2003;104(2):161-6.
41. Shimada H, et al. Clinical significance of
serum vascular endothelial growth factor in esophageal squamous cell carcinoma.
Cancer. 2001;92(3):663-9.
42. Han U, et al. Expressions of p53, VEGF C,
p21: could they be used in preoperative evaluation of lymph node metastasis of
esophageal squamous cell carcinoma? Dis Esophagus. 2007;20(5):379-85.
43. Ren Y, et al. Hepatocyte growth factor
promotes cancer cell migration and angiogenic factors expression: a prognostic
marker of human esophageal squamous cell carcinomas. Clin Cancer Res.
2005;11(17):6190-7.
44. Kimura H, et al. Preoperative serum vascular
endothelial growth factor-C (VEGF-C) levels predict recurrence in patients with
esophageal cancer. Anticancer Res. 2008;28(1A):165-9.
45. Ladeira K, et al. Angiogenic factors: role in
esophageal cancer, a brief review. Esophagus. 2018;15:53-8.
46. Guo X, et al. Prognostic value of microvessel density in esophageal squamous cell carcinoma-a
systematic review and meta-analysis. Pathol Res Pract. 2021;227:153644.
47. Denlinger CE, Thompson RK. Molecular basis of
esophageal cancer development and progression. Surg Clin North Am.
2012;92(5):1089-103.
48. Kitadai Y, et al.
Angiogenic switch occurs during the precancerous stage of human esophageal
squamous cell carcinoma. Oncol Rep. 2004;11(2):315-9.
49. Yoo SY, Kwon SM. Angiogenesis and its
therapeutic opportunities. Mediators Inflamm.
2013;2013(1):127170.
50. Takala H, et al. HIF-1α and VEGF are
associated with disease progression in esophageal carcinoma. J Surg Res.
2011;167(1):41-8.
51. Han B, et al. Clinicopathological
significance of heparanase and basic fibroblast
growth factor expression in human esophageal cancer. World J Gastroenterol.
2005;11(14):2188.
52. Deng Y-Z, et al. Connective tissue growth
factor is overexpressed in esophageal squamous cell carcinoma and promotes
tumorigenicity through β-catenin-T-cell factor/Lef signaling. J Biol Chem.
2007;282(50):36571-81.
53. Tachezy M, et al.
CXCR7 expression in esophageal cancer. J Transl Med. 2013;11:1-6.
54. Wang H, et al. Prognostic significance of
lymph node metastasis in esophageal squamous cell carcinoma. Pathol Res Pract.
2017;213(7):842-7.
55. Krzystek-Korpacka
M, et al. Increase in serum platelet-derived growth factor (PDGF)-BB reflects
lymph node involvement in esophageal cancer patients independently from
platelet count. Exp Oncol. 2011.
56. Hussen BM, et al. MicroRNA: A signature for
cancer progression. Biomed Pharmacother. 2021;138:111528.
57. Price C, Chen J. MicroRNAs in cancer biology
and therapy: current status and perspectives. Genes Dis. 2014;1(1):53-63.
58. Calin GA, et al. Frequent deletions and
down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic
lymphocytic leukemia. Proc Natl Acad Sci U S A.
2002;99(24):15524-9.
59. Halkova T, et al.
MicroRNAs in pancreatic cancer: involvement in carcinogenesis and potential use
for diagnosis and prognosis. Gastroenterol Res Pract.
2015;2015(1):892903.
60. Zhang C, et al. Expression profile of
microRNAs in serum: a fingerprint for esophageal squamous cell carcinoma. Clin
Chem. 2010;56(12):1871-9.
61. Li M, et al. Meta-analysis of microRNAs as
potential biomarkers for detecting esophageal carcinoma in Asian populations.
Int J Biol Markers. 2017;32(4):375-83.
62. Yao C, et al. Diagnostic and prognostic value
of circulating microRNAs for esophageal squamous cell carcinoma: a systematic
review and meta-analysis. J Cancer. 2018;9(16):2876.
63. Wang K, et al. Clinical evaluation of 4 types
of microRNA in serum as biomarkers of esophageal squamous cell carcinoma. Oncol
Lett. 2018;16(1):1196-204.
64. Sun H, et al. Diagnostic and prognostic value
of serum miRNA-1290 in human esophageal squamous cell carcinoma. Cancer Biomark. 2019;25(4):381-7.
65. Meiri E, et al. A second-generation
microRNA-based assay for diagnosing tumor tissue origin. Oncologist
2012;17(6):801-12.
66. Santosh B, et al. Non‐coding RNAs: biological
functions and applications. Cell Biochem Funct. 2015;33(1):14-22.
67. Frankish A, et al. GENCODE: reference
annotation for the human and mouse genomes in 2023. Nucleic Acids Res.
2023;51(D1):D942-D9.
68. Jiang Q, et al. TF2LncRNA: identifying common
transcription factors for a list of lncRNA genes from ChIP‐Seq
data. Biomed Res Int. 2014;2014(1):317642.
69. Schmitt AM, Chang HY. Long noncoding RNAs in
cancer pathways. Cancer cell. 2016;29(4):452-63.
70. Song W, Zou S-b. Prognostic role of lncRNA
HOTAIR in esophageal squamous cell carcinoma. Clin Chim Acta. 2016;463:169-73.
71. Beylerli O, et al.
Long noncoding RNAs as promising biomarkers in cancer. Noncoding RNA Res.
2022;7(2):66-70.
72. Arita T, et al. Circulating long non-coding
RNAs in plasma of patients with gastric cancer. Anticancer Res.
2013;33(8):3185-93.
73. Wang W, et al. Serum HOTAIR as a novel
diagnostic biomarker for esophageal squamous cell carcinoma. Mol Cancer. 2017;16:1-5.
74. Hu H-b, et al. Three circulating LncRNA
predict early progress of esophageal squamous cell carcinoma. Cell Physiol Biochem.
2016;40(1-2):117-25.