Prevalence and
clinical significance of saprophytic bacteria in bloodstream infections among
cancer patients
Sheetal Goenka 1, Wanshisha Wanswett 1, Manisha Jain 1 *, Poonam
Loomba 1, Abha Sharma 1, Shivani Tyagi 1
1 Department of Microbiology, GB Pant
Institute of Medical Education and Research (GIPMER), Delhi, India
Corresponding Authors: Manisha Jain
* Email: manisha_jain29@yahoo.com
Abstract
Introduction: Bloodstream infections (BSIs) in cancer patients are associated with
high morbidity and mortality. While common pathogens are well-studied, the role
of saprophytic bacteria in BSIs among this population is less
understood. To investigate the prevalence and clinical significance of
saprophytic pathogens causing BSIs in cancer patients at a tertiary care
center.
Materials and Methods: This retrospective study included all 200 consecutive adult
cancer patients with suspected sepsis over four months. Blood cultures were
processed on an automated BACTEC system. Subculture and identification were
performed using standard microbiological techniques and the Vitek
2 system. Antimicrobial sensitivity was performed as per CLSI guidelines.
Results: The blood culture positivity in these patients was 79% (158/200).
Of the 158 positive blood cultures, 10.1% (16/158) were saprophytic pathogens.
These included Enterococcus avium, Sphingomonas
paucimobilis, Actinomyces meyeri,
Kodamaea ohmeri, Elizabethkingia meningoseptica,
Aeromonas hydrophila, Achromobacter
xylosoxidans, Stenotrophomonas maltophilia,
Pantoea dispersa, and Burkholderia pseudomallei.
The overall 30-day mortality rate for patients with saprophytic pathogen BSIs
was 20%.
Conclusion: Saprophytic bacteria have gained recognition as possible human
pathogens, especially in immunocompromised patients including cancer patients.
Such high-risk patients should be put on empiric antibiotics to improve patient
outcomes till the time clinical significance is established.
Keywords: Bloodstream infection, Saprophytic organism, Cancer patients
Introduction
Bloodstream infections (BSIs) remain a significant
cause of morbidity and mortality in cancer patients, with mortality rates
ranging from 18% to 42% (1-3). It has been known for decades that the
fundamental cause of the life-threatening organ damage seen in sepsis is not
the direct result of the invading organisms but rather the host response to infection(1,2). Additionally, patients who survive sepsis
endure long-term physical, psychological, and cognitive impairment, known as
post-sepsis syndrome (3,4).
Blood culture remains the gold standard for diagnosing
BSI. While common pathogens like Klebsiella pneumoniae and Escherichia
coli are well-recognized in this setting, the role of saprophytic bacteria
in causing BSIs among cancer patients is less understood (5-7).
The immunocompromised state of cancer patients,
coupled with frequent hospitalizations and invasive procedures, creates a
unique environment where typically non-pathogenic organisms can cause severe infections(8-12). A recurring challenge in clinical practice
is distinguishing true pathogens from colonizers and contaminants in blood
cultures. This study aimed to investigate the prevalence and clinical
significance of saprophytic pathogens causing BSIs in cancer patients in a
large tertiary care center.
Material and methods
This retrospective study was
carried out over four months, from January to April 2023 at one of the large
tertiary care referral center.
A total of 200 consecutive patients (age ≥ 18 years) with a confirmed diagnosis
of cancer presenting with signs and symptoms of bloodstream infection were
included in the study. Non-cancer patients or cancer patients with
polymicrobial bloodstream infections or where the clinical significance of the
isolate could not be determined were excluded from the study.
As a routine hospital
protocol, venous blood was taken aseptically from patients clinically suspected
of having bloodstream infections. The blood was inoculated aseptically into the
automated blood culture bottle and incubated using the BACTEC system. Once
flagged positive, the blood culture bottles (PBC) were processed using standard
microbiological techniques. Briefly, direct gram staining was done from PBC
along with subculture on blood agar and MacConkey agar. The plates were
incubated at 37°C, and the next day growth was observed. The colonies were
identified by colony characteristics, gram stain, and biochemical reactions.
Identification was confirmed by the Vitek 2 system (Biomerieux, France). Antimicrobial susceptibility testing
was carried out by the Vitex 2 system as well as manually using the disk
diffusion method and the antibiotics tested were chosen either from the CLSI
guidelines or the available literature where CLSI guidelines was not available.
Statistical Analysis:
Descriptive statistics were used to summarize patient demographics and clinical
characteristics. Categorical variables were presented as frequencies and
percentages. Continuous variables were expressed as means and ranges. Fisher's exact
test was used to compare mortality rates between groups. A p-value <0.05 was
considered statistically significant. All analyses were performed using SPSS
version 25.0 (IBM Corp., Armonk, NY).
Data Collection and
Analysis: Clinical data including patient demographics, cancer type, presenting
symptoms, and treatment outcomes were collected from medical records. The
frequency of saprophytic pathogens was calculated as a percentage of total
isolates.
The data for this study were
collected as part of routine clinical care and were fully anonymized. It is
essential to highlight that all patient data were de-identified to maintain
confidentiality. Personal identifiers were removed prior to data analysis, and
no identifiable information was used in the study. This approach ensured
compliance with patient privacy regulations and ethical standards. There was no
ethical consideration regarding the study.
Results
Out of 200 patients, 60
(30%) were female and 140 (70%) were male. The mean age was 52 years (range:
18-75 years). The most common cancer types were colorectal (25%), lung (20),
and hematological malignancies (15%) as shown in Figure 1.
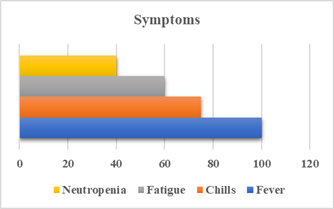
Figure 1. Symptoms prevalence.
A total of 158 organisms
were isolated from 200 patients, indicating a culture positivity rate of 79%.
Common pathogens such as Klebsiella pneumoniae, Acinetobacter baumannii,
Escherichia coli, Enterococcus spp, and
Staphylococcus aureus accounted for 142 (89.9%) of isolates, while 16 (10.1%)
isolates were identified as saprophytic pathogens. This included Enterococcus
avium, Sphingomonas paucimobilis,
Actinomyces meyeri, Kodamaea
ohmeri, Elizabethkingia meningoseptica, Aeromonas hydrophila,
Achromobacter xylosoxidans,
Stenotrophomonas maltophilia, Pantoea
dispersa, Burkholderia pseudomallei as shown in table 1.
The most common presenting
symptoms were fever (100%), chills (75%), and fatigue (60%). Neutropenia was
present in 40% of cases. The overall 30-day mortality rate for patients with
saprophytic pathogen BSIs was 20%, compared to 18% for those with common pathogens
(p=0.42, Fisher's exact test) (Figure 2).
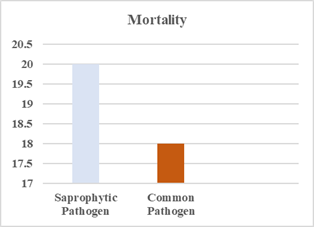
Figure 2. Mortality Rate.
Table 1. Demographic and
Clinical Characteristics of Patients with Saprophytic Pathogen BSIs.
|
Pathogen |
Number of Cases |
Mortality Rate (%) |
|
Enterococcus avium |
1 |
0 |
|
Sphingomonas paucimobilis |
2 |
50 |
|
Actinomyces meyeri |
1 |
0 |
|
Kodamaea ohmeri |
1 |
100 |
|
Elizabethkingia meningoseptica |
2 |
0 |
|
Aeromonas hydrophila |
2 |
0 |
|
Achromobacter xylosoxidans |
2 |
0 |
|
Stenotrophomonas maltophilia |
2 |
0 |
|
Pantoea dispersa |
1 |
0 |
|
Burkholderia pseudomallei |
2 |
50 |
Enterococcus
avium (7-12)
A 60-year-old male with metastatic colon cancer on chemotherapy presented with
fever, lethargy, and unresponsiveness. Blood tests showed leukopenia and
thrombocytopenia with elevated lactate levels. Blood cultures revealed Enterococcus
avium, sensitive to ampicillin, vancomycin, and linezolid, but resistant to
high-level gentamicin, ciprofloxacin, levofloxacin, and erythromycin.
Vancomycin was administered, resulting in clinical improvement and resolution
of fever over 7 days. Enterococcus avium is a rare pathogen in humans,
often found in birds, and requires prompt diagnosis and treatment, especially
in immunocompromised patients.
Sphingomonas paucimobilis (13-17)
Case 1: A 37-year-old male with metastatic pancreatic cancer had a fever and
chills. Sphingomonas paucimobilis,
sensitive to ciprofloxacin, ceftazidime, ceftriaxone, meropenem, and imipenem,
was isolated from blood cultures. Treatment with ceftriaxone led to clinical
improvement and sterile follow-up cultures.
Case 2: A 20-year-old male
with meningioma presented with fever and headache. Blood culture grew Sphingomonas paucimobilis,
sensitive to ceftazidime and ceftriaxone but resistant to ciprofloxacin,
meropenem, and imipenem. The patient succumbed to septicemia
despite ceftriaxone treatment.
Actinomyces meyeri (18-19)
A 46-year-old woman with cervical cancer on chemotherapy presented with fever
and hypotension. Blood culture initially showed no growth but later identified Actinomyces
meyeri. Sensitive to penicillin, ciprofloxacin,
and amoxicillin-clavulanic acid, she was treated with penicillin but left the
hospital against medical advice after 3 days of worsening condition. Actinomyces
meyeri is a rare pathogen, typically part of
polymicrobial infections, and is often underdiagnosed.
Kodamaea ohmeri (20-22)
A 28-year-old male with colorectal adenocarcinoma and traumatic pancreatic
injury presented with abdominal distention, poor appetite, and weight loss. Kodamaea ohmeri, sensitive to
amphotericin B, itraconazole, and voriconazole but resistant to fluconazole,
was isolated. Despite voriconazole therapy, the patient’s condition
deteriorated rapidly, leading to death. Kodamaea
ohmeri is an emerging opportunistic pathogen with
high mortality rates.
Elizabethkingia meningoseptica (23-24)
Case 1: A 58-year-old male with meningioma had a fever and weakness. Elizabethkingia meningoseptica,
sensitive to ciprofloxacin, amikacin, and minocycline, was isolated.
Ciprofloxacin treatment led to significant clinical improvement.
Case 2: A 63-year-old male
with metastatic lung cancer had respiratory symptoms and fever. Blood culture
grew Elizabethkingia meningoseptica,
sensitive to ciprofloxacin and resistant to gentamicin. Ciprofloxacin treatment
resolved symptoms and follow-up cultures were negative. Elizabethkingia
meningoseptica is an emerging nosocomial pathogen
often associated with high mortality in cancer patients.
Aeromonas hydrophila (25-26)
Case 1: A 62-year-old male with chronic lymphoid leukemia
presented with fever and dizziness. Aeromonas hydrophila,
sensitive to multiple antibiotics, was treated with meropenem, leading to
symptom resolution.
Case 2: An HIV-positive
patient with colorectal cancer and a recent leg injury presented with fever and
elevated leukocytes. Aeromonas hydrophila,
sensitive to multiple antibiotics including trimethoprim-sulfamethoxazole, was
treated successfully. Aeromonas hydrophila is
increasingly recognized as a significant pathogen in immunocompromised
patients.
Achromobacter xylosoxidans (27-28)
Case 1: A 64-year-old male with colon cancer and a 58-year-old female with
pancreatic cancer, both with type 2 diabetes, presented with fever and chills. Achromobacter xylosoxidans,
sensitive to ciprofloxacin, was isolated. Both patients responded well to
ciprofloxacin treatment. Achromobacter xylosoxidans can cause significant infections,
particularly in immunocompromised individuals.
Stenotrophomonas maltophilia (29-30)
Case 1: A 67-year-old male with sigmoid adenocarcinoma had persistent fever and
cough. Stenotrophomonas maltophilia, treated
with trimethoprim-sulfamethoxazole and levofloxacin, showed clinical
improvement.
Case 2: A 60-year-old male
with glioblastoma presented with fever and altered mental status. Stenotrophomonas
maltophilia was treated with
trimethoprim-sulfamethoxazole and ceftazidime with gradual improvement. Stenotrophomonas
maltophilia is challenging to diagnose and manage
but responds well to trimethoprim-sulfamethoxazole.
Pantoea dispersa (31)
A 35-year-old chronic alcoholic with liver cirrhosis presented with abdominal
pain, fever, and vomiting. Pantoea dispersa, sensitive to minocycline, was treated
effectively, leading to the resolution of symptoms. Pantoea
dispersa, while less virulent, can cause
significant infections in immunocompromised individuals.
Burkholderia pseudomallei (32-34)
Case 1: A 57-year-old male with colon cancer improved significantly with
imipenem therapy after isolation of Burkholderia
pseudomallei.
Case 2: A 43-year-old female
with pulmonary tuberculosis and ovarian cancer succumbed to septic shock
despite aggressive treatment. Burkholderia pseudomallei, endemic to tropical regions, poses a high
mortality risk and highlights the need for early detection and preventive
measures.
This study shows that
saprophytic pathogens account for a notable proportion of bloodstream
infections in cancer patients, emphasizing the need for accurate identification
and targeted treatment, particularly for high-mortality organisms like Kodamaea ohmeri and
Burkholderia pseudomallei.
Discussion
Our study reveals that saprophytic pathogens account
for a significant proportion (10.1%) of bloodstream infections (BSIs) in cancer
patients, highlighting the importance of considering these organisms in the
differential diagnosis of BSIs, especially in immunocompromised hosts. This
finding is consistent with recent literature that has increasingly recognized
the role of opportunistic pathogens in causing severe infections in vulnerable
populations (1,2).
The prevalence of saprophytic pathogens in our study
(10.1%) is slightly higher than that reported by Rega et al (6), who found a
7.5% prevalence of unusual bacterial isolates in BSIs among Ethiopian cancer
patients (3). This difference might be attributed to variations in geographical
location, patient population, or improvements in diagnostic techniques. Our
findings underscore the need for clinicians to maintain a high index of
suspicion for atypical pathogens in cancer patients presenting with signs of BSI.
Of particular note was the isolation of Kodamaea ohmeri and
Burkholderia pseudomallei,
both associated with high mortality rates. K. ohmeri,
once considered a benign organism, has emerged as an opportunistic pathogen
capable of causing invasive infections in immunocompromised individuals (4).
Similarly, B. pseudomallei, the causative
agent of melioidosis, is increasingly recognized as a significant threat to
immunocompromised patients, particularly in endemic regions (5). These findings
align with recent global surveillance data that highlight the growing
importance of emerging pathogens in healthcare-associated infections (6).
Our statistical analysis revealed no significant
difference in 30-day mortality rates between patients with saprophytic pathogen
BSIs and those with common pathogens (20% vs. 18%, p=0.42). This finding is
intriguing and contrasts with some previous studies that have reported higher
mortality rates associated with unusual pathogens (7,8).
The successful treatment of some cases with targeted
antimicrobial therapy in our study demonstrates the importance of prompt and
accurate identification of these pathogens. This observation is supported by
recent literature emphasizing the critical role of rapid diagnostics and
appropriate antimicrobial stewardship in managing BSIs, particularly those
caused by unusual pathogens (9,10)
Our study also highlights the challenges in
distinguishing true pathogens from colonizers or contaminants, particularly in
the case of saprophytic organisms. This dilemma is well-recognized in clinical
microbiology and emphasizes the need for careful interpretation of blood
culture results in the context of the patient's clinical presentation (13,14).
The implementation of clinical decision support systems and machine learning
algorithms shows promise in aiding clinicians in this complex decision-making
process (15).
The high rate of neutropenia (40%) observed in our
cohort of cancer patients with BSIs is consistent with previous studies and
underscores the vulnerability of this population to opportunistic infections
(16,17). Recent research has focused on strategies to prevent and manage
infections in neutropenic cancer patients, including the use of prophylactic
antimicrobials and immunomodulatory agents (18,19). Our findings support the
need for tailored approaches to infection prevention and management in this high-risk
group.
While our study provides valuable insights into the
prevalence and clinical significance of saprophytic pathogens in
cancer-associated BSIs, it has several limitations. As a single-center study with a relatively small sample size,
particularly for saprophytic pathogen infections, the statistical power of our
comparisons is limited. This may have prevented us from detecting significant
differences in outcomes between groups. Additionally, the short duration of the
study precluded analysis of seasonal variations in pathogen distribution and
potential confounding factors that may influence patient outcomes. These
limitations highlight the need for larger, multi-center
studies with longer follow-up periods to more comprehensively characterize the
epidemiology and clinical impact of saprophytic pathogen BSIs in cancer
patients.
Despite these limitations, our study contributes to
the growing body of evidence highlighting the importance of saprophytic
pathogens in BSIs among cancer patients. The findings underscore the need for
heightened awareness among clinicians, improved diagnostic strategies, and
tailored antimicrobial approaches for managing these infections. Future
research should focus on developing rapid diagnostic tools specifically
targeted at identifying unusual pathogens, as well as exploring novel
therapeutic strategies for managing infections caused by these emerging
organisms.
Conclusion
Our study demonstrates that saprophytic pathogens
account for a significant proportion (10.1%) of BSIs in cancer patients, with
no statistically significant difference in mortality rates compared to common pathogens(1-6,25). These findings underscore the importance
of considering these organisms in the differential diagnosis of BSIs,
especially in immunocompromised hosts. Further large-scale, multicenter
studies are needed to better understand the epidemiology and clinical impact of
saprophytic pathogen BSIs in cancer patients.
In the present study, the patients were started on
early empiric therapy and almost 75% of the patients responded to the
treatment. The response to treatment in the present study reiterates that the
presence of saprophytic bacteria from cases of BSI should not be ignored in
cancer patients. It would be worthwhile to start the patient on early empiric
treatment till the time a repeat blood culture is sent for confirmation of the
clinical significance of these isolates. Raising awareness among healthcare providers
about the potential for such infections is crucial to ensure timely diagnosis
and intervention.
Author
contribution
ShG: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation,
Visualization, Writing original draft, Writing review & editing. WW: Writing review & editing, Resources, Software,
Data curation, Methodology, Project administration, Software, Validation. MJ: Conceptualization, Formal analysis, Investigation,
Methodology, Project administration, Resources, Supervision, Validation,
Visualization, Writing original draft, Writing review
& editing. PL: Conceptualization, Formal analysis,
Funding acquisition, Investigation, Methodology, Project
administration, Resources. Ash: Conceptualization, Data curation, Formal
analysis, Funding acquisition, Investigation,
Methodology, Project administration, Resources, Software, Supervision. ShT: Investigation, administration.
Conflict
of interest
The
authors declare that they have no competing interests.
Funding
There
is no funding agency involved in this research.
Ethical
approval
This
is a retrospective study and there is no ethical consideration related to
paper. The data for this study was collected as part of routine clinical care
and was fully anonymized. All patient data were de-identified to maintain
confidentiality. Personal identifiers were removed prior to data analysis, and
no identifiable information was used in the study. This approach ensures
compliance with patient privacy regulations and ethical standards.
References
1. Mu S,
Xiang H, Wang Y, et al. The pathogens of secondary infection in septic patients
share a similar genotype to those that predominate in the gut. Crit Care. 2022;26:68.
2. McCreery
RJ, Florescu DF, Kalil AC. Sepsis in Immunocompromised Patients Without Human
Immunodeficiency Virus. J Infect Dis. 2020;221(2):156–65.
3. Van der Slikke EC, An AY, Hancock REW, Bouma HR. Exploring the
pathophysiology of post-sepsis syndrome to identify therapeutic opportunities. EBioMedicine. 2020;61:103044.
4. Singer M,
Deutschman MC. Improving the prevention, diagnosis and clinical management of
sepsis. WHO. 2017 Jan 9.
5. Viscoli C. Bloodstream Infections: The peak of the iceberg.
Virulence. 2016;7(3):248–51.
6. Rega B,
Wolde-Amanuel Y, Adane K, Belay E, Abubeker A, Asrat D, et al. Saprophytic
bacterial isolates causing bloodstream infections in Ethiopian patients with
cancer. Infect Agent Cancer. 2017;12:40.
7. Patel R,
Keating MR, Cockerill III FR, Steckelberg JM. Bacteremia
Due to Enterococcus avium. Clin Infect Dis. 1993;17(6):1006–11.
8. Bhagat B,
Shah T, Roistacher K, Glatt AE. Enterococcus avium
infection. Infect Dis Clin Pract1996;5(8):502–3.
9. Mirzoyev Z, Anavekar N, Wilson F,
Uslan D, Baddour L, Mookadam F. Enterococcus avium
endocarditis. Scand J Infect Dis. 2004;36(11–12):876–8.
10. Osoba AO, Kutub H, Waliuddin A.
Enterococcus avium: An unusual cause of cerebral abscess. Neurosci
J. 2005;10(4):297–300.
11. Shin BS,
Jang NY, Park HE, Cheung YD, Park HS. A Case of Enterococcus Avium Colitis with
Endoscopic Finding. Gastroenterol Hepatol. 2017;8(1):00267.
12. Abo-Zed A,
Hegazy S, Phan T. Detection of Enterococcus avium in a case of urinary tract
infection and haematuria. Access Microbiol.
2022;4(5):000349.
13. Laupland KB, Paterson DL, Stewart AG, Edwards F, Harris PN.
Sphingomonas paucimobilis
bloodstream infection. Int J Infect Dis. 2022 Jun;119:172-177.
14. Tito E,
Ahmad A, Gongolli J, Issack W, Johnson A. Sphingomonas paucimobilis Bacteremia in a Patient with Retropharyngeal Abscess. Cureus. 2022;14(5).
15. Nandy S,
Dudeja M, Das AK, Tiwari R. Community Acquired Bacteremia
by Sphingomonas paucimobilis:
Two Saprophytic Case Reports. J Clin Diagn Res.
2013;7(12):2947–9.
16. Cheong HS,
Wi YM, Moon SY, Kang CI, Son JS, Ko KS, et al. Clinical features and treatment
outcomes of infections caused by Sphingomonas paucimobilis. Infect Control Hosp Epidemiol.
2008;29(10):990–2.
17. Lin JN, Lai
CH, Chen YH, Lin HL, Huang CK, Chen WF, et al. Sphingomonas
paucimobilis bacteremia in
humans: 16 case reports and a literature review. J Microbiol
Immunol Infect. 2010;43(1):35–42.
18. Apothéloz C, Regamey C.
Disseminated infection due to Actinomyces meyeri:
case report and review. Clin Infect Dis. 1996;22(4):621–5.
19. Verrienti G, Megliola G, Antonaci E, Gisotti A, Raccagni C. Actinomyces meyeri
Causing Cerebral Abscess in a Patient on Methotrexate: A Saprophytic Case
Report and Systematic Review of the Literature. Cureus.
2023;15(6):e41204.
20. Diallo K,
Lefevre B, Cadelis G, Gallois JC, Gandon F, Nicolas
M. A case report of fungemia due to Kodamaea ohmeri. BMC Infect Dis. 2019;19(1):1-3.
21. Zhou M, Li
Y, Kudinha T, Xu Y, Liu Z. Kodamaea ohmeri as an Emerging Human Pathogen: A Review and Update.
Front Microbiol. 2021;12:736582.
22. Zhou M, Yu
S, Kudinha T, Xiao M, Wang H, Xu Y, et al. Identification and antifungal
susceptibility profiles of Kodamaea ohmeri based on a seven-year multicenter
surveillance study. Infect Drug Resist. 2019;12:1657–64.
23. Li Y, Liu
T, Shi C, et al. Epidemiological, clinical, and laboratory features of patients
infected with Elizabethkingia meningoseptica
at a tertiary hospital in Hefei City, China. Front Public Health. 2022;10:964046.
24. Jean SS,
Lee WS, Chen FL, Ou TY, Hsueh PR. Elizabethkingia meningoseptica: an important emerging pathogen causing
healthcare-associated infections. J Hosp Infect. 2014;86(4):244–9.
25. Hughes WT,
Armstrong D, Bodey GP, et al. Guidelines for the Use of Antimicrobial Agents in
Neutropenic Patients with Cancer. Clin Infect Dis. 2002;34(6):730–51.
26. Okumura K,
Shoji F, Yoshida M, Mizuta A, Makino I, Higashi H. Severe sepsis caused by
Aeromonas hydrophila in a patient using tocilizumab:
a case report. J Med Case Rep. 2011;5(1):1-3.
27. Isler B,
Kidd TJ, Stewart AG, Harris P, Paterson DL. Achromobacter
infections and treatment options. Antimicrob Agents Chemother. 2020;64(11):10-128.
28. Swenson CE,
Sadikot RT. Achromobacter
respiratory infections. Ann Am Thorac Soc.
2015;12(2):252-8.
29. Said MS, Tirthani E, Lesho E. Stenotrophomonas Maltophilia.
[Updated 2023 Jun 12]. In: StatPearls [Internet].
Treasure Island (FL): StatPearls Publishing; 2024
Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK572123/
30. Cho SY, Lee
DG, Choi SM, Park C, Chun HS, Park YJ, et al. Stenotrophomonas maltophilia bloodstream infection in patients with
hematologic malignancies: a retrospective study and in vitro activities of
antimicrobial combinations. BMC Infect Dis. 2015;15:1-8.
31. Asai N,
Koizumi Y, Yamada A, Sakanashi D, Watanabe H, Kato H,
et al. Pantoea dispersa bacteremia in an immunocompetent patient: a case report and
review of the literature. J Med Case Rep. 2019;13(1):1-5.
32. Barman P,
Sidhwa H, Shirkhande PA. Melioidosis: A Case Report.
J Glob Infect Dis. 2011;3(2):183–6.
33. Meumann EM, Cheng AC, Ward L, Currie BJ. Clinical features
and epidemiology of melioidosis pneumonia: results from a 21-year study and
review of the literature. Clin Infect Dis. 2012;54(3):362-9.
34. Hussin A,
Nor Rahim MY, Dalusim F, Shahidan
MA, Nathan S, Ibrahim N. Improving the clinical recognition, prognosis, and
treatment of melioidosis through epidemiology and clinical findings: The Sabah
perspective. PLoS Negl Trop
Dis. 2023;17(10):e0011696.