Targeting multiple cancers: exploring
the potential role of stem cells in treating 10 distinct types
Munawar Ali1*,
Syeda Areej Imran1, Hafiza Malaika Choudhary1, Laiba
Batool1, Amna Amin1, Muhammad Umair Aslam1,
Hafiz Muhammad Sultan1
1 Institute of Biological Sciences, Khwaja Fareed University of
Engineering and Information Technology, Rahim Yar Khan, Punjab Pakistan
Corresponding
Authors: Munawar Ali
* Email: jammunawarali.456@gmail.com
Abstract
Stem cell research has gained significant prominence due to its
therapeutic potential in addressing diseases that are difficult to treat with
conventional therapies, particularly cancer. Cancer remains a global health
crisis responsible for one in six deaths worldwide and is characterized by
uncontrolled cell growth, metastasis, and a generalized loss of growth control.
Traditional cancer treatments, including surgery, radiation, and chemotherapy,
have limitations, such as damaging healthy cells and tissues, and are often
associated with cancer recurrence and metastasis. In response to these
challenges, stem cell technology has emerged as a promising frontier, offering
novel approaches to target and eliminate cancer cells while potentially
reducing the side effects associated with conventional therapies. This review
explores the biochemical properties of stem cells and their potential
applications in treating ten distinct types of cancer. We analyze each cancer
type to understand the potential use of stem cells in treatment. The article
aims to contribute the growing body of knowledge and provide insights into the
future directions of stem cell research in oncology.
Keywords: Stem Cell Therapy, Cancer Treatment, Oncology, Multi-cancer Therapy,
Regenerative Medicine
Graphical abstract

Introduction
In
recent years, stem cell research has gained significant attention due to its
potential therapeutic applications in addressing challenging diseases, many of
which remain largely untreatable by conventional therapies, particularly
cancer. Cancer represents a significant global health issue, accounting for one
in every six deaths across the globe (1). This intricate and destructive
disease, marked by the unregulated growth and multiplication of cells,
continues to pose a substantial challenge to health systems worldwide. This
condition arises from abnormal cell proliferation resulting from genetic mutations
(2). Cancer cells possess the
capability to proliferate without limit and circulate to various regions of the
body through the process of metastasis. This represents a highly intricate
series of medical conditions that advance progressively, resulting in a widespread
loss of regulatory control over growth. For many decades, patients had limited
options for cancer treatment, primarily consisting of surgery, radiation
therapy, and chemotherapy, either as standalone therapies or in various
combinations (3). Radiation therapy has the
potential to harm healthy cells, organs, and tissues (4). Conversely, while chemotherapy has
significantly decreased morbidity and mortality rates, it is important to note
that nearly all chemotherapeutic agents adversely affect healthy cells,
particularly those that are rapidly dividing and growing (5). While conventional treatment
approaches can significantly decrease tumor size, the recurrence and spread of
cancer remain persistent challenges.
During
this time, cancer treatments have been the subject of more than half of all
ongoing medical treatment research worldwide. Cancer remains a leading cause of mortality worldwide, despite
advancements in traditional treatments such as surgery, chemotherapy, and
radiation therapy. However, stem cell technology, emerging as a
promising frontier, complements traditional cancer treatments by offering novel
approaches to target and eliminate cancer cells while potentially reducing the
side effects associated with chemotherapy and radiation (6). Researchers are harnessing the
distinctive characteristics of stem cells, including their ability to
self-renew and differentiate, to create innovative treatment approaches that
are more precise, effective, and less harmful. Stem cell therapies have the
potential to specifically target cancer cells by modifying stem cells to
transport therapeutic agents directly to tumor locations, thereby reducing harm
to surrounding healthy tissues. This review article explores the biochemical
underpinnings of stem cells and delves into the exciting possibilities of stem
cell technology in combating various types of cancer. We will navigate the
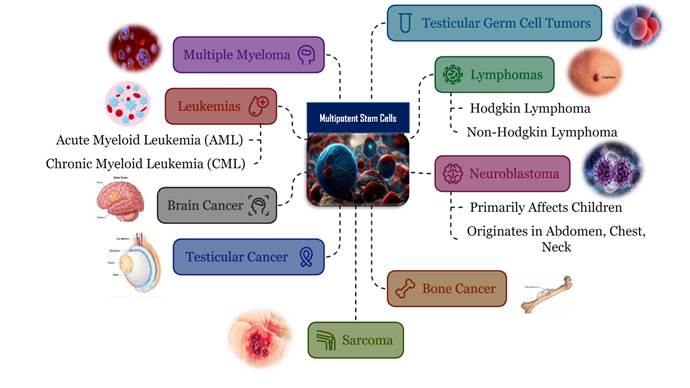
potential applications of stem cells in treating ten distinct types of cancers,
including: myeloid leukemia (acute & chronic), brain tumor, lymphoma,
multiple myeloma, germ cell tumor, testicular cancer, osteosarcoma (bone
cancer), Neuroblastoma (develops from immature nerve cells), and sarcoma
(develops in soft tissues).
Acute myeloid leukemia (AML) and chronic myeloid
leukemia (CML) are both bone marrow cancers characterized by abnormal white
blood cell production (7). Brain cancer is a deadly disease with limited treatment options (8). Testicular cancer is a
rare male cancer with two main types: seminomas and non-seminomas (9). Osteosarcoma is a prevalent bone cancer, primarily affecting
adolescents (10). Lymphoma is a cancer of lymphatic tissue classified into Hodgkin and
non-Hodgkin types, originating from B, T, or natural killer cells.
Multiple myeloma is a cancer of plasma cells causing various health issues (11). Testicular germ cell tumors (TGCTs) originate from abnormal testicular
stem cell development (12). One kind of cancer that arises in immature nerve cells is called
Neuroblastoma. It primarily affects children and can originate in various parts
of the body, including the abdomen, chest, and neck (13). Sarcoma is a cancer that arises in soft tissues, such as muscle, fat,
blood vessels, or connective tissue. It can occur in people of all ages and
often affects the arms, legs, or trunk (14).
This
journey will also acknowledge the current challenges and ongoing research
efforts aimed at translating this potential into effective and widely
accessible clinical applications. As we embark on this exploration, one thing
remains certain: stem cell technology has the potential to reshape the future
of cancer treatment, presenting a future filled with renewed hope for patients
worldwide. It's important to note that stem cell therapy for cancer is still
under development. Several stem cell therapies are available; however, the
majority remain in experimental phases, are expensive, or raise ethical
concerns (15). While these characteristics hold
promise, researchers are working on overcoming challenges such as assuring the
safety and effectiveness of stem cell therapy. Despite the challenges, stem
cell research offers a ray of hope for the future of cancer treatment. By
utilizing the specialized characteristics of stem cells, scientists are
developing novel approaches to combat this complex disease.
1. Therapeutic Potential of Stem Cells in Cancer Treatment
A
stem cell is defined as a type of cell that possesses the capacity for
continuous division and the ability to differentiate into diverse other cell
types or tissues. Stem cells serve as a vital reservoir within the body’s
cellular system. In instances where there is a deficiency of specific cell
types, stem cells can transform into those required cells, such as liver and
kidney cells. Additionally, since blood cells and muscle cells lack the
capability to divide and produce new cells, stem cells also play a crucial role
in producing blood and muscle cells within the body. On the basis of origin or
formation type, stem cells are commonly divided into Embryonic Stem Cells,
Adult Stem Cells, and Induced Pluripotent Stem Cells. Based on the level of
differentiation (potency), stem cells can be categorized as totipotent,
pluripotent, multipotent, oligopotent and unipotent stem cells (16).
Stem
cells possess the ability to self-renew through a process of continuous
division over time. This characteristic allows them to serve as a potentially
limitless source of cells for therapeutic purposes. In cancer treatment, this
could be beneficial for replacing diseased or damaged blood-forming stem cells
in bone marrow with healthy ones, often from a donor. Stem cells have the
capability to generate similar stem cells. These cells possess the unique
ability to differentiate into specific cell types within the body, such as
blood cells, lung cells, and kidney cells, thereby contributing to the
maintenance of cellular equilibrium (17). Furthermore, stem cells perform a
crucial function in stimulating organs by providing necessary cellular support.
They can replace aged, dying, or damaged cells, ensuring that organs
consistently receive fresh cells to operate effectively.
Stem
cells can be modified to transport therapeutic agents directly to tumor
locations, thereby reducing harm to surrounding healthy tissues (18). This can be accomplished through
several approaches, including the genetic alteration of stem cells to generate
and release therapeutic proteins (such as anti-cancer medications and
cytokines), encapsulating drugs within the stem cells, or employing stem cells
as carriers to deliver nanoparticles or alternative drug delivery systems to
the tumor microenvironment. Stem cells possess the capability to be modified in
order to strengthen the immune system's response to cancer cells through a
variety of mechanisms (19). These cells can be designed to
produce cytokines or other immune-activating substances that stimulate immune
cells, including T cells and natural killer cells, thus enhancing the overall
immune response. Furthermore, stem cells can be genetically altered to express
tumor-specific antigens, which can initiate an immune reaction against cancer
cells. This approach may also encompass the use of stem cells to develop
adoptive cell therapies, such as chimeric antigen receptor T cells (CART
cells), which are specifically tailored to identify and eliminate cancer cells (20).
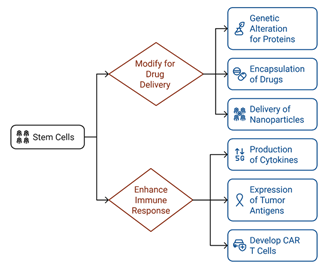
Figure 1. The role of stem cells in targeted drug delivery and immune
modulation for cancer treatment.
Stem
cell therapy is commonly referred to as regenerative medicine (21). Depending on the type of stem
cell, they can differentiate into various specialized cell types. In some
cancers, healthy tissue is damaged during treatment. Stem cells could
potentially be used to regenerate healthy tissue, such as liver or bone, after
cancer treatment. Certain stem cells exhibit immunomodulatory characteristics,
indicating their ability to affect the immune system's reaction. This could be
beneficial for suppressing tumor growth by stimulating the immune system to
attack cancer cells, reducing inflammation caused by cancer or its treatment (22). Due to these characteristics, stem
cells hold immense potential in many diseases, especially cancer treatment.
Their distinctive capability for self-renewal and differentiation into multiple
cell types offers novel therapeutic avenues. By targeting cancer stem cells,
researchers aim to eradicate the root cause of tumor growth. Additionally, stem
cells can be engineered to deliver therapeutic agents directly to cancer sites
or to boost the mmune system's anti-tumor response (18). While significant challenges
remain, such as the ethical considerations associated with the utilization of
embryonic stem cells in cancer treatment, the potential of stem cell-based
therapies to improve patient outcomes is driving extensive research and
clinical development. But our question is, can stem cell-based therapies offer
a more personalized approach to cancer treatment in the future?
2. The Role of Stem Cells in Cancer Treatment: Addressing Diverse
Cancer Types and Challenges
Because
of their exceptional capacity to self-renew, differentiate into a various cell
types, and potentially target malignant cells, stem cells have become a viable
tool in the treatment of several forms of cancer. This review delves into the
therapeutic potential of stem cells in addressing a spectrum of cancers. Each
type of cancer presents unique challenges and complexities, which we have
thoroughly examined and discussed. By exploring the diverse applications of
stem cells in these cancer types, this study underscores the potential of stem
cell-based therapies to revolutionize cancer treatment. However, challenges
such as tumor heterogeneity (23), immune suppression (24), and ethical considerations must be
carefully addressed to realize the full potential of this approach. Tumor
heterogeneity refers to the genetic and phenotypic variations present among
cancer cells within a single tumor. These differences can significantly reduce
the overall effectiveness of treatments. To enhance the efficacy of targeted
therapies and ensure consistent treatment results, it is essential to recognize
and address the issue of heterogeneity (25). Tumors generate an
immunosuppressive environment that reduces the effectiveness of
immunotherapies, including those that involve stem cells. Mechanisms like
cytokine modulation and the function of regulatory T-cells allow tumors to
escape detection by the immune system. Addressing immune suppression to improve
therapeutic results continues to be a key area of research (26).
3.1. Acute myeloid
leukemia
A
study by Hahn et al. (2015) revealed that approximately 14,000 new cases of
Acute Myeloid Leukemia (AML) were identified, and 10,000 deaths were recorded
in the United States during the year 2013 (27). Acute Myeloid Leukemia (AML) is a
bone marrow-derived malignancy leading to the rapid proliferation of abnormal
white blood cells. It is caused by genetic alteration that affects
hematopoietic stem cells and causes an excess production of malignant clonal
myeloid stem cells. Abnormal leukemic cells cause disruptions in the normal
synthesis of blood cells, which results in bleeding, infections, and
exhaustion. While extra-medullary manifestations may arise, the primary cause
of the disease lies in abnormalities related to hematopoietic cell production.
A small percentage of cases can be linked to prior chemotherapy or chemical
exposures; however, the vast majority are attributed to chromosomal anomalies
or mutations in single genes, without an apparent cause (28). Hematopoietic stem cells, a
specific type of stem cell, have been used to treat acute myeloid leukemia.
Hematopoietic stem cells are employed in the treatment of Acute Myeloid
Leukemia (AML) by replacing the affected bone marrow with healthy stem cells
that can yield normal blood cells and may also eradicate cancerous cells. This
approach aims to reset the immune system and achieve long-term remission by
eradicating leukemia cells and restoring healthy hematopoiesis.
3.2.
Chronic
Myeloid Leukemia
A
bone marrow-derived malignancy known as chronic myeloid leukemia (CML) is
distinguished by a high concentration of white blood cells and the Philadelphia
chromosome. Edition et al. (2017) reported the incidence of CML to be 1-2 cases
per 100,000 adults (29). However, it's important to note
that the incidence of CML can vary depending on factors such as age, geographic
location, and population demographics This disease progresses through three
stages: chronic, accelerated, and blast crisis. Each stage has a higher
severity and more difficult treatment options. A study by Champlin et al.
(2011) revealed that treatment for CML involves the use of stem cell therapy,
including allogeneic hematopoietic stem cell transplantation (HSCT), in which
the patient receives transplants of healthy stem cells from a donor (30). This technique is thought to be
the only viable approach for CML. Because of the possibility of reintroducing
leukemic cells, autologous stem cell transplantation—which employs the
patient's own stem cells—is less prevalent in CML patients. However, patients
who do not have a compatible donor may still undergo this procedure. Moreover,
stem cell transplants are frequently used in conjunction with targeted therapy
like tyrosine kinase inhibitors (TKIs), which include medications like
imatinib, dasatinib, and nilotinib to lessen the impact of the disease and
sustain recovery (31) (32). Identifying an appropriate donor,
controlling graft-versus-host disease (GVHD), and coping with a high risk of
infection and other problems after transplantation are some of the difficulties
associated with HSCT. Clinical results for CML patients receiving stem cell
therapy have greatly improved, and many of them have achieved long-term
recovery, especially when HSCT is carried out when the illness is still in its
chronic stage (33).
Table 1. Comparison of Acute Myeloid Leukemia (AML) and Chronic Myeloid Leukemia
(CML).
|
Sr No |
Feature |
Acute Myeloid Leukemia (AML) |
Chronic Myeloid Leukemia (CML) |
|
1 |
Definition |
Malignant
disorder of bone marrow leading to rapid proliferation of abnormal white
blood cells |
Chronic bone
marrow disorder characterized by increased white blood cells and the
Philadelphia chromosome |
|
2 |
Incidence |
Approximately 14,000 new cases and 10,000 deaths in the US in
2013 |
1-2 cases per 100,000 adults |
|
3 |
Disease
Stages |
Single acute
phase |
Chronic,
accelerated, and blast crisis phases |
|
4 |
Genetic
Abnormalities |
Various chromosomal abnormalities and single gene mutations |
Presence of the Philadelphia chromosome |
|
5 |
Treatment
Approach |
Primarily
hematopoietic stem cell transplantation (HSCT) |
HSCT and
targeted therapy (TKIs) |
|
6 |
Challenges |
Finding suitable donor, graft-versus-host disease |
Finding suitable donor, graft-versus-host disease, resistance to
TKIs |
3.3.
Brain
tumor
One
of the hardest tumors to cure is brain cancer (34). The latest worldwide cancer
statistics issued by the World Health Organization (WHO) in 2020 indicate that
brain tumors make up around 1.6% of all reported cases and account for 2.5% of
all tumor-related deaths. Brain tumors may be classified into two categories:
primary and secondary brain cancer. Primary brain cancer arises from brain
cells and develops inside the central nervous system (CNS), often without
spreading to other parts of the body outside the CNS. Secondary brain cancer
arises and spread from outside the central nervous system (CNS), namely from
organs such as lung, skin, breast, colon, and kidney (35). Brain cancer that is very
malignant, aggressive, and usually fatal is called glioblastoma multiforme
(GBM). The frequent and fast recurrence of GBM leads to a low 5-year survival
rate of just 4%, despite the use of gold-standard therapies such as temozolomide-based
chemotherapy and radiotherapy. The Blood Brain Barrier (BBB) makes it difficult
to treat brain tumors effectively because it prevents medications from getting
to the afflicted region. Lengel et al. (2020) investigated that a unique
therapeutic method to treat brain damage has been the use of exosomes derived
from stem cells (36). Stem cells are important for the
treatment of brain tumors because they show promise for both specific cancer
treatment and regenerative therapies. Cancer stem cells (CSCs) are hypothesized
to be present in brain tumors and to be the cause of the tumor's proliferation
and resistance to conventional therapies. Targeting these CSCs can result in
more successful treatments, while regular stem cells can be used in
regenerative therapies to repair damage caused by malignancies or their
treatment (37).
3.4.
Testicular
Cancer
Testicular
carcinoma is an uncommon form of tumor that makes up only 1% of all cancers in
males; it is also known as testicular germ cell carcinoma (TGCC). 50% of all
TGCC are seminomas, while the remaining 50% are non-seminomas. A study by
Popovic et al. (2015) revealed that most testicular germ cell cancers (TGCC)
originate from the gonads, whereas approximately 5% originate from extragonadal
areas along the body's mid-line, such as the retroperitoneum, mediastinum, or
brain (38). Various surgical and hormonal
interventions are available for TGCC treatment; however, in recent years, there
has been a significant concentration on stem cell therapy (39). The notion of cancer stem cells
(CSCs) states that CSCs are accountable for the growth, invasion, and spread of
tumors (40). Spermatogonial stem cells (SSCs)
are a type of stem cell found in the testis that perform a crucial role in the
process of spermatogenesis, which is necessary for male fertility (41). Stem cells can be obtained from
numerous resources, such as bone marrow, peripheral blood, dental pulp, hair
follicles, and adipose tissue, with adipose tissue being considered one of the
more accessible sources for isolating stem cells to treat these types of
cancers.
3.5.
Osteosarcoma
(Bone cancer)
Osteosarcoma
is the predominant tumor of the bone, primarily affecting individuals in the
infant and adolescent age groups. Eaton et al. (2021) estimated that reports of
osteosarcoma cases in youngsters are 4.4 per million annually (42). Lin et al. (2021) investigated
that osteosarcoma primarily develops from the metaphysis of long bones,
primarily affecting the proximal and distal ends of the humerus and femur (43). The substantial side effects and
high dose levels necessary for the effectiveness are the constraints of this
commonly used anticancer drug (44). Mesenchymal stem cells (MSCs) are
found locally next to tumor tissues and may interact directly with
malignancies, according to recent investigations. Through genetic engineering
or spontaneous transformation, human bone marrow mesenchymal stromal cells
(BMSCs) can support OS development (45). According to accumulating data,
Hinoi et al. (2024) suggest that targeting OSCs may be a successful tactic for
enhancing OS treatment (46).
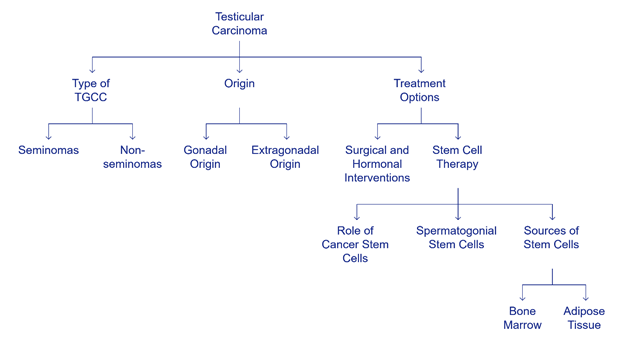
Figure 2. Testicular germ cell tumors: origin, treatment, and the role of
stem cells.
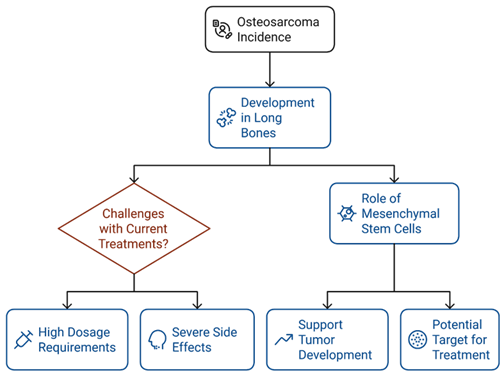
Figure 3. Osteosarcoma: a pediatric bone cancer with significant therapeutic
challenges.
3.6.
Lymphoma
Lymphoma
constitutes a diverse group of malignant neoplasms of lymphocytes that includes
lymphatic tissue, bone marrow, or extra-nodal sites. Lymphoma develops due to
its expansion through extra-nodal sites by direct invasion or by circulatory
flow to the spleen, liver, lungs, or bone marrow. He et al. (2023) reported
that the primary classification of lymphoma is obtained from B-cell, T-cell, or
natural killer cell origin (47). On the other hand, Hazani and
Isaac (2019) noted that lymphoma commonly presents as painless adenopathy, with
symptoms such as fever, severe weight loss, or night sweats often occurring in
the critical stage of the disorder (48). It is conventionally categorized
usually as non-Hodgkin and Hodgkin lymphoma.
Hodgkin
lymphoma (HL) is an abnormal B-cell lymphoma that is specifically distinguished
by some malignant cells, such as cancerous Reed-Sternberg cells in an
inflammatory environment (49). Non-Hodgkin lymphoma (n-HL) is a
diverse group of malignant diseases emerging from the cells of the immune
system, such as lymphoid tissue (50). Almost all non-Hodgkin lymphomas
emerge from mature B lymphocytes, while few of them are produced from T
lymphocytes or natural killer (NK) cells. According to Chu et al. (2023),
several risk factors for both lymphomas include genetics, viral infections,
immune-deficiency disorders (such as HIV), physical relationships, and
environmental hazards (51).
Repeated
or refractory disorder can be efficiently handled or treated by high-dose
chemotherapy following autologous stem cell transplantation (ASCT), yet there
is a notable portion of some patients that relapse after the treatment.
Allogeneic stem cell transplantation (All-SCT) can be utilized for those
patients who have repeated disorders or those who have failed ASCT (52). Therefore, the development of some
novel agents, such as antibody-based therapies and checkpoint inhibitors, has
remarkably increased the outgrowth of patients with HL or n-HL that repeats
after ASCT. Probably, the development of some latest drugs in the first-line
setting will efficiently enhance prolonged outcomes of ASCT (53).
Table 2. List of some novel agents/antibody-based therapies or checkpoint
inhibitors for the treatment of Hodgkin’s lymphoma and non-Hodgkin’s lymphoma
after ASCT.
|
Lymphomas |
Antibody-based therapies |
Checkpoint inhibitors |
Reference |
|
Hodgkin’s
lymphoma |
Brentuximab
Vedotin (BV) AFM13 ADCT-301 Camidanlumab
Tesirine (Cami) |
Nivolumab Pembrolizumab Histone
deacetylase inhibitors Chimeric
antigen receptor (CAR) T-cells |
(54) |
|
Non-Hodgkin’s
lymphoma |
Rituximab Brentuximab vedotin (BV) Alemtuzumab Ofatumumab |
Pembrolizumab (Anti-PD1) Nivolumab (Anti-PD1) Atezolizumab (Anti-PD-L1) Ipilimumab (Anti-CTLA-4) |
(55) |
3.7.
Multiple
myeloma
Multiple
myeloma is a hemostatic malignancy of plasma cells that develops monoclonal
immunoglobulin in an abnormal way, arising from the bone marrow. Rajkumar
(2018) reported that this disorder usually presents with hypercalcemia, renal
failure, anemia, and a large number of infections (56). Nearly all patients with multiple
myeloma arise from an asymptomatic pre-malignant stage called monoclonal
gammopathy of undetermined significance (MGUS) that is described by the
appearance of a monoclonal protein. Various risk factors are involved, such as
high levels of monoclonal protein, the presence of plasma cells in the bone
marrow, or a high percentage of IgA monoclonal protein (57).
The
establishment of high-dose therapy after autologous stem cell transplantation
(ASCT) is an ordinary treatment for young patients with early stages of
multiple myeloma (58). Advanced therapies for these
patients as a medicinal application conveyed before ASCT or after ASCT, such as
induction, consolidation, and maintenance treatment in order to eliminate tumor
overburden and minimize attainable infections on normal hematopoietic cells. An
enlargement of immunomodulatory drugs such as thalidomide, lenalidomide,
pomalidomide, and monoclonal antibodies like daratumumab, along with
second-generation proteasome inhibitors for example, carfilzomib, bortezomib
etc., gave rise to the remarkable advancement in the survey of patients having
multiple myeloma (MM), appropriate for autologous stem cell transplantation
(ASCT) (59).
3.8.
Testicular
germ cell tumor
Human
germ cell tumors (GCTs) are believed to emerge from stem cells of premature
embryos and the germ line that are present in the gonads (ovaries or testes) as
well as in extragonadal sites, where primordial germ cells (PGCs) are found
during embryogenesis (60). Dieckmann et al. (2018) found that
testicular germ cell tumor (TGCT) is the most frequent malignant GCT in adults
or young men, accounting for almost 95%, with variations observed across
different geographical regions (61). The phenomena of TGCT are a
complex procedure where many molecular deformities give rise to its evolution,
characterized by some environmental or hormonal factors. Spermatogenesis is a
basic principle to a male’s progress and prolongation of fertility. TGCTs occur
due to the failure of normal maturation of testicular stem cells (TSCs)
controlled by clinical procedure, where TSCs do not encounter exact
spermatogenic differentiation yet convert into intratubular germ cell neoplasia
(IGCN) or carcinoma in situ (CIS) that presents as the precursor cells for
first stage TGCTs (62).
Differentiation
therapy has the ability, just like chemotherapy, to utilize non-cytotoxic
advanced procedures to minimize tumor development, such as pluripotent
embryonal stem cells, particularly influence the separation of Cancer Stem
Cells (CSCs), whereby decreasing the possibility for metastasis that leads to
repetition of the disorder (40). Specific applications utilized in
order to produce CSC-targeted therapies that include some drugs such as
salinomycin, an antibiotic that diminishes mammary development of tumors, as
well as Thioridazine, an antipsychotic, which particularly influences the
depletion of CSCs without changing normal hematopoietic stem cells.
3.9.
Neuroblastoma
Morgenstern
et al. (2013) reported that neuroblastoma (NB) is a solid tumor that
constitutes 6% of all pediatric cancers and is the most prevalent cancer
diagnosed in infants (63). Neural crest cells are the
progenitors of the sympathetic nervous system and the source of neuroblastoma.
Neuroblastoma begins in the adrenal glands but also finds its way in nerve
tissues along the spine, chest, abdomen, or pelvis. Systemic spread and
recurrent relapses are clinical features of NB disease development, with a
short survival timeline (1st relapse in 18 months, 2nd relapse in 8.7 months,
and 3rd relapse in 3.8 months). Obtaining a cure after a relapse of progressive
non-communicable bone disease is extremely difficult due to the disease's
heterogeneous behavior (64). There are many different treatment
options for neuroblastoma, and these generally combine several methods.
Surgical procedures, chemotherapy, radiation therapy, and immunotherapy are
examples of conventional treatments. Surgery by alone may be curative in
low-risk patients. Intense chemotherapy is usually required for intermediate-
and high-risk neuroblastoma, and the tumor may occasionally be surgically
removed after treatment. When a tumor is incurable or there is leftover
disease, radiation therapy is frequently used to treat it. Additionally,
Kushner et al. (2005) highlighted that immunotherapy—especially using anti-GD2
antibodies—has emerged as a key component of neuroblastoma treatment, aiding in
the more precise targeting and elimination of cancer cells (65).
Two
consecutive autologous stem cell transplants (tandem ASCT) increased event-free
survival rates in children with high-risk neuroblastoma when compared to a
single transplant, according to a noteworthy research conducted by the
Children's Oncology Group (COG) (66).
Although long-term results are still being investigated, preliminary
trials employing CAR-T cells and stem cell-derived treatments show promise.
These treatments aim to lower recurrence rates and enhance patients' quality of
life. Using stem cells in conjunction with precision medicine techniques like
gene editing and immunomodulation to customize therapies to each patient's
requirements is becoming more and more supported by research (67). The use of genetically modified
natural killer T cells to combat neuroblastoma was investigated in a recent
clinical experiment. Early-phase studies of this immunotherapeutic strategy
have demonstrated preliminary safety and effectiveness, giving optimism for
further advancements. To produce NK cells for targeted treatment, researchers
are looking into using human induced pluripotent stem cells (iPSCs).
Scalability and immunological compatibility are two benefits of iPSCs that are
essential for treating pediatric malignancies (68). Neuroblastoma treatment has been
revolutionized by stem cell technology, particularly for high-risk cases. Mora
(2022) indicated that following intense chemotherapy, autologous stem cell
transplantation (ASCT), which involves harvesting and reinfusing hematopoietic
stem cells from the patient's bone marrow or blood, is now considered standard
therapy (69). By mending the damage that
chemotherapy has caused to the bone marrow, this mechanism improves survival
rates and permits greater chemotherapy dosages. Furthermore, continuing studies
on mesenchymal stem cells (MSCs) have shown promise in directly delivering
targeted therapeutics to tumors, potentially leading to less toxic and more
effective therapy options.
3.10.
Sarcoma
Sarcoma
is a diverse group of cancers that arise in the bones and soft tissues,
involving fat, muscle, blood vessels, deep skin tissues, and nerves (14). It accounts for about 1% of adult
cancers and 15% of pediatric cancers, making it relatively rare but challenging
due to its diverse presentation and aggressive nature. Sarcomas are broadly
classified into two main categories: bone sarcomas, such as osteosarcoma and
Ewing sarcoma, and soft tissue sarcomas, including liposarcoma, leiomyosarcoma,
and angiosarcoma (70). These tumors have the capability
to arise in any region of the body; in spite of this, they are mainly located
in the arms, legs, and trunk. Bindal et al. (1994) highlighted that the
clinical manifestations of sarcoma vary based on the tumor's size and location,
often leading to a delayed diagnosis. Additionally, sarcomas are known for
their ability to metastasize, particularly to the lungs, which complicates
treatment and significantly worsens prognosis (71). Standard treatment options for
sarcoma typically involve a combination of surgery, radiation therapy, and
chemotherapy. Surgical resection with clear margins is the primary approach for
localized sarcoma, while radiation therapy is often used to shrink the tumor
before surgery or to eliminate residual disease postoperatively. Chemotherapy
is more commonly employed for high-grade or metastatic sarcomas, although its
effectiveness can be limited (72).
Stem
cell technology has recently emerged as a promising avenue for improving
sarcoma treatment outcomes. For high-risk cases, autologous stem cell
transplantation (ASCT) following high-dose chemotherapy has been explored as a
means to restore the patient's bone marrow function and allow for more
aggressive chemotherapy regimens (73). Moreover, ongoing research into
mesenchymal stem cells (MSCs) offers hope for the development of novel
therapies that could directly target sarcoma cells, reduce tumor growth, and
enhance the precision of treatment while minimizing damage to surrounding
healthy tissues. The ability of mesenchymal stem cells (MSCs) to directly
transport anti-cancer drugs to tumors is being investigated. In order to stop
tumor growth, researchers are investigating whether modified MSCs can target
sarcoma sites and release therapeutic chemicals like cytokines or gene-editing
tools (74). Clinical research is looking into
how stem cell-derived therapies may be used in conjunction with more
conventional treatments like radiation and chemotherapy. By decreasing tumor
recurrence and promoting tissue healing after therapy, this strategy seeks to
improve the effectiveness of current techniques (3). Regenerative therapy is another
area of attention for stem cell research in sarcoma. Stem cells are being
investigated for fostering tissue regeneration and reducing harm from invasive
therapies after surgically excising malignancies. The genetic manipulation of
stem cells for precise sarcoma targeting is still being refined in lab
research. Among these initiatives is altering the cells to withstand immune
suppression techniques frequently used by sarcoma tumor (75). Although still in the experimental
stages, these approaches represent a potential paradigm shift in the management
of this complex and often deadly group of cancers.
Table 3. Overview of Stem Cell-Based Therapies for Different Cancer Types.
|
Sr No |
Cancer Type |
Stem Cell Type(s) |
Potency |
Clinical Applications |
References |
|
1 |
Acute Myeloid Leukemia |
Hematopoietic |
Multipotent |
Bone marrow transplantation |
(76) |
|
2 |
Chronic
Myeloid Leukemia |
Hematopoietic |
Multipotent |
Bone
marrow transplantation (allogenic) |
(77) |
|
3 |
Brain Tumor |
Mesenchymal, Neural Stem Cells |
Multipotent |
Tumor targeting, immune modulation, tissue repair |
(78) |
|
4 |
Testicular
Cancer |
Spermatogonial
stem cells |
Multipotent |
Tumor
replacement, immune modulation, tissue repair |
(79) |
|
5 |
Osteosarcoma |
Mesenchymal stem Cells |
Multipotent |
Bone regeneration, tumor suppression |
(80) |
|
6 |
Lymphoma |
Hematopoietic,
Mesenchymal |
Multipotent |
Immune
modulation, tumor targeting, tissue repair |
(81) |
|
7 |
Multiple Myeloma |
Autologous Hematopoietic stem cells |
Multipotent |
Bone marrow regeneration, immune modulation |
(82) |
|
8 |
Germ
Cell Tumor |
Embryonic
Induced Pluripotent Stem Cells |
Pluripotent |
Tumor
replacement, differentiation therapy |
(83) |
|
9 |
Neuroblastoma |
Neural Stem Cells, Mesenchymal Stem Cells |
Multipotent |
Tumor targeting, immune modulation, tissue repair |
(84) |
|
10 |
Sarcoma |
Mesenchymal
Stem Cells |
Multipotent |
Tumor
suppression, tissue regeneration |
(85) |
4. Challenges
There's
a potential risk of uncontrolled cell growth and tumor formation if
transplanted stem cells are not properly controlled. Stem cells, particularly
induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs), have
the ability to differentiate uncontrollably, leading to the formation of tumors
themselves (86). Ensuring the safety and minimizing
side effects of stem cell-based treatments is crucial for clinical translation. Stem cell therapy is often expensive
and may be inaccessible to patients due to high treatment costs. Ensuring that
these treatments are cost-effective and widely available is an important
challenge (87). Variability in stem cell sources, isolation methods, culture
conditions, and differentiation protocols can lead to inconsistencies in cell
quality and therapeutic outcomes. Immune
rejection continues to pose a considerable obstacle in the field of stem cell
transplantation therapies. The immune system may recognize stem cells as
foreign and reject them, particularly if the stem cells are derived from donors
or are genetically modified (88). This poses a challenge for the
long-term effectiveness of stem cell-based cancer therapies. Strategies to
mitigate immune rejection, such as immunosuppression. The long-term effects of stem cell therapy in cancer treatment are
not yet fully known. Monitoring patients over time to assess the safety and
efficacy of these treatments is a critical aspect of their development (89).
4.
Future
prospects
The
exploration of stem cells in cancer treatment holds significant promise, yet
much remains to be discovered and refined before these therapies can be fully
realized in clinical practice. One of the key future directions is the
enhancement of targeted delivery systems, ensuring that stem cells can
accurately locate and treat cancer cells without affecting healthy tissues.
Lotfi et al. (2023) suggested that advances in nanotechnology and gene editing,
such as CRISPR, may play an essential role in enhancing the precision and
efficacy of stem cell-based therapies (90). Another important area of future
work involves overcoming the risks associated with stem cell therapy,
particularly the potential for tumorigenicity and immune rejection (91). Developing safer stem cell lines,
along with improved methods for controlling stem cell differentiation, will be
critical for the success of these treatments. The convergence of immunotherapy
and stem cell therapy presents significant potential for transforming the
landscape of cancer treatment (92). By harnessing the power of the
immune system and the regenerative potential of stem cells, this synergistic
approach offers novel strategies to combat cancer. The use of engineered stem
cells is another method that is being actively pursued (93). New approaches have been developed
in light of our growing knowledge of the molecular mechanisms underlying stem
cell self-renewal and proliferation as well as the identification of additional
genes governing stem cell differentiation and proliferation. Nanomedicine
is another strategy that has lately been considered as a potential method in
cancer treatments (94).
Conclusion
In
conclusion, stem cell research represents a transformative approach in the
fight against cancer, offering new hope for patients facing this devastating
disease. As cancer remains one of the prominent causes of death globally,
traditional therapies like surgery, radiation, and chemotherapy, though
effective in many cases, often fall short due to their inability to fully
eradicate cancer cells and prevent recurrence. Stem cell-based therapies
present a promising alternative, with their unique capability to identify and
eradicate cancer stem cells, potentially reducing the risk of relapse and
metastasis. The potential of stem cells to distinguish into multiple cell
types, along with their ability for self-renewal, opens new avenues for cancer
treatment, particularly for types of cancer that have limited treatment
options. However, significant challenges remain, including the risk of
tumorigenicity, immune rejection, and the high cost of treatment. Despite these
challenges, the future of stem cell-based cancer therapy is bright. Advances in
technology, such as gene editing and targeted delivery systems, are likely to
overcome many of the current obstacles, bringing us closer to acknowledging the
full potential of these therapies. The integration of stem cell therapy with
existing treatments, such as immunotherapy, could revolutionize cancer care,
offering more effective and less harmful treatment options. As research
continues to advance, stem cell-based therapies may soon become a cornerstone
of cancer treatment, offering new hope for millions of patients worldwide.
Author
contribution
MA, LB,
and SAI design the study. MA, SAI, HMC, LB, and AA, wrote the first
draft of the manuscript. MUA wrote
a section of the manuscript. LB and FEM made tables. HMS revised the manuscript. All the authors contributed to the
article and approved the submitted version.
Conflict
of interest
There
is no Conflicts of interest/competing interests.
Funding
There
is no funding.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward
E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians.
2011;61(2):69–90.
2. Hanselmann RG, Welter C. Origin of cancer:
cell work is the key to understanding cancer initiation and progression.
Frontiers in Cell and Developmental Biology. 2022;10:787995.
3. Debela DT, Muzazu SG, Heraro KD, Ndalama
MT, Mesele BW, Haile DC, et al. New approaches and procedures for cancer
treatment: Current perspectives. SAGE open medicine. 2021;9:20503121211034366.
4. Abshire D, Lang MK. The evolution of
radiation therapy in treating cancer. In Elsevier; 2018. p. 151–7.
5. Yazbeck V, Alesi E, Myers J, Hackney MH,
Cuttino L, Gewirtz DA. An overview of chemotoxicity and radiation toxicity in
cancer therapy. Advances in Cancer Research. 2022;155:1–27.
6. Hmadcha A, Martin-Montalvo A, Gauthier BR,
Soria B, Capilla-Gonzalez V. Therapeutic potential of mesenchymal stem cells
for cancer therapy. Frontiers in bioengineering and biotechnology. 2020;8:43.
7. Dörmer P, Lau B, Wilmanns W. Kinetics of
bone marrow cell production in human acute and chronic myeloid leukemias.
Leukemia Research. 1980;4(2):231–7.
8. Ningaraj N, Salimath B, Sankpal U, Perera
R, Vats T. Targeted brain tumor treatment-current perspectives. Drug Target
Insights. 2007;2:117739280700200008.
9. Giona S. The epidemiology of testicular
cancer. Exon Publications. 2022;107–16.
10. Ottaviani G, Jaffe N. The epidemiology of
osteosarcoma. Pediatric and adolescent osteosarcoma. 2010;3–13.
11. de Leval L, Jaffe ES. Lymphoma
classification. The Cancer Journal. 2020;26(3):176–85.
12. Baroni T, Arato I, Mancuso F, Calafiore R,
Luca G. On the origin of testicular germ cell tumors: from gonocytes to
testicular cancer. Frontiers in endocrinology. 2019;10:343.
13. Hildebrandt T, Traunecker H. Neuroblastoma: a
tumour with many faces. Current Paediatrics. 2005;15(5):412–20.
14. Vodanovich DA, Choong PF. Soft-tissue
sarcomas. Indian journal of orthopaedics. 2018;52:35–44.
15. Lo B, Parham L. Ethical issues in stem cell
research. Endocrine reviews. 2009;30(3):204–13.
16. Alison MR, Poulsom R, Forbes S, Wright NA. An
introduction to stem cells. The Journal of Pathology: A Journal of the
Pathological Society of Great Britain and Ireland. 2002;197(4):419–23.
17. Klimczak A, Kozlowska U. Mesenchymal stromal
cells and tissue‐specific progenitor cells: Their role in tissue homeostasis.
Stem cells international. 2016;2016(1):4285215.
18. Chulpanova DS, Kitaeva KV, Tazetdinova LG,
James V, Rizvanov AA, Solovyeva VV. Application of mesenchymal stem cells for
therapeutic agent delivery in anti-tumor treatment. Frontiers in pharmacology.
2018;9:259.
19. Clara JA, Monge C, Yang Y, Takebe N.
Targeting signalling pathways and the immune microenvironment of cancer stem
cells—a clinical update. Nature reviews clinical oncology. 2020;17(4):204–32.
20. Depil S, Duchateau P, Grupp S, Mufti G,
Poirot L. ‘Off-the-shelf’allogeneic CAR T cells: development and challenges.
Nature reviews Drug discovery. 2020;19(3):185–99.
21. Tatullo M, Gargiulo IC, Dipalma G, Ballini A,
Inchingolo AM, Paduanelli G, et al. Stem cells and regenerative medicine. In:
Translational systems medicine and oral disease. Elsevier; 2020. p. 387–407.
22. Wada N, Gronthos S, Bartold PM.
Immunomodulatory effects of stem cells. Periodontology 2000.
2013;63(1):198–216.
23. El-Sayes N, Vito A, Mossman K. Tumor
heterogeneity: a great barrier in the age of cancer immunotherapy. Cancers.
2021;13(4):806.
24. Holthof LC, Mutis T. Challenges for
immunotherapy in multiple myeloma: bone marrow microenvironment-mediated immune
suppression and immune resistance. Cancers. 2020;12(4):988.
25. Syga S, Jain HP, Krellner M, Hatzikirou H,
Deutsch A. Evolution of phenotypic plasticity leads to tumor heterogeneity with
implications for therapy. PLOS Computational Biology. 2024;20(8):e1012003.
26. Ma Q, Long W, Xing C, Chu J, Luo M, Wang HY,
et al. Cancer stem cells and immunosuppressive microenvironment in glioma.
Frontiers in immunology. 2018;9:2924.
27. Hahn A, Giri S, Yaghmour G, Martin MG. Early
mortality in acute myeloid leukemia. Leukemia research. 2015;39(5):505–9.
28. Pelcovits A, Niroula R. Acute myeloid
leukemia: a review. Rhode Island medical journal. 2020;103(3):38–40.
29. Edition S, Edge S, Byrd D. AJCC cancer
staging manual. AJCC cancer staging manual. 2017;
30. Champlin R, Jabbour E, Kebriaei P, Anderlini
P, Andersson B, De Lima M. Allogeneic stem cell transplantation for chronic
myeloid leukemia resistant to tyrosine kinase inhibitors. Clinical Lymphoma
Myeloma and Leukemia. 2011;11:S96–100.
31. Hochhaus A, Larson RA, Guilhot F, Radich JP,
Branford S, Hughes TP, et al. Long-term outcomes of imatinib treatment for
chronic myeloid leukemia. New England Journal of Medicine. 2017;376(10):917–27.
32. JS T. The structure of Dasatinib (BMS-354825)
bound to activated ABL kinase domain elucidates its inhibitory activity against
imatinib-resistant ABL mutants. Cancer Res. 2006;66:5790–7.
33. Nair AP, Barnett MJ, Broady RC, Hogge DE,
Song KW, Toze CL, et al. Allogeneic hematopoietic stem cell transplantation is
an effective salvage therapy for patients with chronic myeloid leukemia
presenting with advanced disease or failing treatment with tyrosine kinase
inhibitors. Biology of Blood and Marrow Transplantation. 2015;21(8):1437–44.
34. Chen X, Momin A, Wanggou S, Wang X, Min HK,
Dou W, et al. Mechanosensitive brain tumor cells construct blood-tumor barrier
to mask chemosensitivity. Neuron. 2023;111(1):30–48.
35. Zhao Y, Yue P, Peng Y, Sun Y, Chen X, Zhao Z,
et al. Recent advances in drug delivery systems for targeting brain tumors.
Drug delivery. 2023;30(1):1–18.
36. Lengel D, Sevilla C, Romm ZL, Huh JW,
Raghupathi R. Stem cell therapy for pediatric traumatic brain injury. Frontiers
in neurology. 2020;11:601286.
37. Bradshaw A, Wickremsekera A, Tan ST, Peng L,
Davis PF, Itinteang T. Cancer stem cell hierarchy in glioblastoma multiforme.
Frontiers in surgery. 2016;3:21.
38. Popovic L, Matovina-Brko G, Popovic M,
Petrovic D, Cvetanovic A, Vukojevic J, et al. High dose chemotherapy with stem
cell support in the treatment of testicular cancer. World Journal of Stem
Cells. 2015;7(11):1222.
39. Ismail HY, Hussein S, Shaker NA, Rizk H,
Wally Y. Stem cell treatment trials for regeneration of testicular tissue in
laboratory animals. Reproductive Sciences. 2023;30(6):1770–81.
40. Loehr AR, Pierpont TM, Gelsleichter E, Galang
AMD, Fernandez IR, Moore ES, et al. Targeting cancer stem cells with
differentiation agents as an alternative to genotoxic chemotherapy for the
treatment of malignant testicular germ cell tumors. Cancers. 2021;13(9):2045.
41. Liu HC, Xie Y, Deng CH, Liu GH. Stem
cell-based therapies for fertility preservation in males: current status and
future prospects. World journal of stem cells. 2020;12(10):1097.
42. Eaton BR, Schwarz R, Vatner R, Yeh B, Claude
L, Indelicato DJ, et al. Osteosarcoma. Pediatric blood & cancer.
2021;68:e28352.
43. Lin J, Wang X, Wang X, Wang S, Shen R, Yang
Y, et al. Hypoxia increases the expression of stem cell markers in human
osteosarcoma cells. Oncology Letters. 2021;21(3):1–1.
44. Kucerova L, Altanerova V, Matuskova M,
Tyciakova S, Altaner C. Adipose tissue–derived human mesenchymal stem cells
mediated prodrug cancer gene therapy. Cancer research. 2007;67(13):6304–13.
45. Huang Y, Liu W, He B, Wang L, Zhang F, Shu H,
et al. Exosomes derived from bone marrow mesenchymal stem cells promote
osteosarcoma development by activating oncogenic autophagy. Journal of bone
oncology. 2020;21:100280.
46. Hinoi E, Osumi R, Sugihara K, Yoshimoto M,
Tokumura K, Tanaka Y. Role of proteoglycan synthesis genes in osteosarcoma stem
cells. Frontiers in Oncology. 2024;14:1325794.
47. He X, Gao Y, Li Z, Huang H. Review on natural
killer/T‐cell lymphoma. Hematological Oncology. 2023;41(2):221–9.
48. Hazani A, Isaac B. Unexplained Fever In
Hematologic Disorders Section 1. Benign Hematologic Disorders. In: Unexplained
fever. CRC Press; 2019. p. 189–208.
49. Piris MA, Medeiros LJ, Chang KC. Hodgkin
lymphoma: a review of pathological features and recent advances in
pathogenesis. Pathology. 2020;52(1):154–65.
50. Singh R, Shaik S, Negi BS, Rajguru JP, Patil
PB, Parihar AS, et al. Non-Hodgkin’s lymphoma: A review. Journal of family
medicine and primary care. 2020;9(4):1834–40.
51. Chu Y, Liu Y, Fang X, Jiang Y, Ding M, Ge X,
et al. The epidemiological patterns of non-Hodgkin lymphoma: global estimates
of disease burden, risk factors, and temporal trends. Frontiers in Oncology.
2023;13:1059914.
52. Kopińska A, Koclęga A, Wieczorkiewicz-Kabut
A, Woźniczka K, Kata D, Włodarczyk M, et al. Allogeneic Stem Cell
Transplantation for Relapsed and Refractory Hodgkin Lymphoma: Real World
Experience of a Single Center. Pathology and Oncology Research. 2021;27.
53. Vassilakopoulos TP, Asimakopoulos JV,
Konstantopoulos K, Angelopoulou MK. Optimizing outcomes in relapsed/refractory
Hodgkin lymphoma: a review of current and forthcoming therapeutic strategies.
Therapeutic advances in hematology. 2020;11:2040620720902911.
54. Randall MP, Spinner MA. Optimizing treatment
for relapsed/refractory classic hodgkin lymphoma in the era of immunotherapy.
Cancers. 2023;15(18):4509.
55. El Boghdadly Z, Sarwar S, Lustberg ME.
Infectious Challenges with Novel Antibody–Based Therapies. Current Infectious
Disease Reports. 2021;23(7):10.
56. Rajkumar SV. Multiple myeloma: 2018 update on
diagnosis, risk‐stratification, and management. American journal of hematology.
2018;93(8):1091–110.
57. Morrison T, Booth RA, Hauff K, Berardi P,
Visram A. Laboratory assessment of multiple myeloma. Advances in clinical
chemistry. 2019;89:1–58.
58. Bazarbachi AH, Al Hamed R, Malard F,
Harousseau JL, Mohty M. Relapsed refractory multiple myeloma: a comprehensive
overview. Leukemia. 2019;33(10):2343–57.
59. Bozic B, Rutner J, Zheng C, Ruckser R, Selimi
F, Racz K, et al. Advances in the treatment of relapsed and refractory multiple
myeloma in patients with renal insufficiency: novel agents, immunotherapies and
beyond. Cancers. 2021;13(20):5036.
60. Oosterhuis JW, Looijenga LH. Human germ cell
tumours from a developmental perspective. Nature Reviews Cancer.
2019;19(9):522–37.
61. Dieckmann KP, Richter-Simonsen H, Kulejewski
M, Ikogho R, Zecha H, Anheuser P, et al. Testicular germ-cell tumours: a
descriptive analysis of clinical characteristics at first presentation.
Urologia internationalis. 2018;100(4):409–19.
62. Batool A, Karimi N, Wu XN, Chen SR, Liu YX.
Testicular germ cell tumor: a comprehensive review. Cellular and Molecular Life
Sciences. 2019;76:1713–27.
63. Morgenstern DA, Baruchel S, Irwin MS. Current
and future strategies for relapsed neuroblastoma: challenges on the road to
precision therapy. Journal of pediatric hematology/oncology. 2013;35(5):337–47.
64. Lau L, Tai D, Weitzman S, Grant R, Baruchel
S, Malkin D. Factors influencing survival in children with recurrent
neuroblastoma. Journal of pediatric hematology/oncology. 2004;26(4):227–32.
65. Kushner BH, Cohn SL, Matthay KK, Cheung NKV,
La Quaglia MP, Haas-Kogan DA, et al. Treatment of neuroblastoma. In:
Neuroblastoma. Springer; 2005. p. 123–92.
66. Park JR, Kreissman SG, London WB, Naranjo A,
Cohn SL, Hogarty MD, et al. Effect of tandem autologous stem cell transplant vs
single transplant on event-free survival in patients with high-risk
neuroblastoma: a randomized clinical trial. Jama. 2019;322(8):746–55.
67. Zheng M, Kumar A, Sharma V, Behl T, Sehgal A,
Wal P, et al. Revolutionizing pediatric neuroblastoma treatment: unraveling new
molecular targets for precision interventions. Frontiers in Cell and
Developmental Biology. 2024;12:1353860.
68. Alidadi M, Barzgar H, Zaman M, Paevskaya OA,
Metanat Y, Khodabandehloo E, et al. Combining the induced pluripotent stem cell
(iPSC) technology with chimeric antigen receptor (CAR)-based immunotherapy:
recent advances, challenges, and future prospects. Frontiers in Cell and
Developmental Biology. 2024;12:1491282.
69. Mora J. Autologous stem-cell transplantation
for high-risk neuroblastoma: Historical and critical review. Cancers.
2022;14(11):2572.
70. Dancsok AR, Asleh-Aburaya K, Nielsen TO.
Advances in sarcoma diagnostics and treatment. Oncotarget. 2017;8(4):7068.
71. Bindal RK, Sawaya RE, Leavens ME, Taylor SH,
Guinee VF. Sarcoma metastatic to the brain: results of surgical treatment.
Neurosurgery. 1994;35(2):185–91.
72. Gronchi A, Colombo C, Raut CP. Surgical
management of localized soft tissue tumors. Cancer. 2014;120(17):2638–48.
73. Schaaf M, Reiser M, Borchmann P, Engert A,
Skoetz N. High‐dose therapy with autologous stem cell transplantation versus
chemotherapy or immuno‐chemotherapy for follicular lymphoma in adults. Cochrane
Database of Systematic Reviews. 2012;(1).
74. Spalato-Ceruso M, Ghazzi NE, Italiano A. New
strategies in soft tissue sarcoma treatment. Journal of Hematology &
Oncology. 2024;17(1):76.
75. Sánchez-Molina S, Figuerola-Bou E,
Sánchez-Margalet V, de la Cruz-Merino L, Mora J, de Álava Casado E, et al.
Ewing sarcoma meets epigenetics, immunology and nanomedicine: moving forward
into novel therapeutic strategies. Cancers. 2022;14(21):5473.
76. Ball ED, Mills LE, Cornwell G 3d, Davis BH,
Coughlin CT, Howell AL, et al. Autologous bone marrow transplantation for acute
myeloid leukemia using monoclonal antibody-purged bone marrow. 1990;
77. Barnett MJ, Eaves AC, Phillips GL. An
overview of bone marrow transplantation for chronic myeloid leukemia. CMAJ:
Canadian Medical Association Journal. 1990;143(3):187.
78. Kwon S, Yoo KH, Sym SJ, Khang D. Mesenchymal
stem cell therapy assisted by nanotechnology: a possible combinational
treatment for brain tumor and central nerve regeneration. International journal
of nanomedicine. 2019;5925–42.
79. Ibtisham F, Honaramooz A. Spermatogonial stem
cells for in vitro spermatogenesis and in vivo restoration of fertility. Cells.
2020;9(3):745.
80. Chang X, Ma Z, Zhu G, Lu Y, Yang J. New
perspective into mesenchymal stem cells: Molecular mechanisms regulating
osteosarcoma. Journal of bone oncology. 2021;29:100372.
81. Casiraghi F, Remuzzi G, Abbate M, Perico N.
Multipotent mesenchymal stromal cell therapy and risk of malignancies. Stem
cell reviews and reports. 2013;9(1):65–79.
82. Lu Y, Zheng C, Zhang W, Liu X, Zhou Z, Wang
Z, et al. Characterization of the biological and transcriptomic landscapes of
bone marrow-derived mesenchymal stem cells in patients with multiple myeloma.
Cancer Cell International. 2024;24(1):116.
83. Niu Z, Hu Y, Chu Z, Yu M, Bai Y, Wang L, et
al. Germ‐like cell differentiation from induced pluripotent stem cells (iPSCs).
Cell Biochemistry and Function. 2013;31(1):12–9.
84. Veschi V, Verona F, Thiele CJ. Cancer stem
cells and neuroblastoma: characteristics and therapeutic targeting options.
Frontiers in endocrinology. 2019;10:782.
85. Lye KL, Nordin N, Vidyadaran S, Thilakavathy
K. Mesenchymal stem cells: from stem cells to sarcomas. Cell Biology
International. 2016;40(6):610–8.
86. Afify SM, Seno M. Conversion of stem cells to
cancer stem cells: undercurrent of cancer initiation. Cancers. 2019;11(3):345.
87. Ratcliffe E, Thomas RJ, Williams DJ. Current
understanding and challenges in bioprocessing of stem cell-based therapies for
regenerative medicine. British medical bulletin. 2011;100(1):137.
88. Haworth R, Sharpe M. Accept or reject: the
role of immune tolerance in the development of stem cell therapies and possible
future approaches. Toxicologic Pathology. 2021;49(7):1308–16.
89. Goldring CE, Duffy PA, Benvenisty N, Andrews
PW, Ben-David U, Eakins R, et al. Assessing the safety of stem cell
therapeutics. Cell stem cell. 2011;8(6):618–28.
90. Lotfi M, Morshedi Rad D, Mashhadi SS, Ashouri
A, Mojarrad M, Mozaffari-Jovin S, et al. Recent Advances in CRISPR/Cas9
Delivery Approaches for Therapeutic Gene Editing of Stem Cells. Stem cell
reviews and reports. 2023;19(8):2576–96.
91. Bruttel VS, Wischhusen J. Cancer stem cell
immunology: key to understanding tumorigenesis and tumor immune escape?
Frontiers in immunology. 2014;5:360.
92. Ali M, Shabbir K, Ali S, Mohsin M, Kumar A,
Aziz M, et al. A New Era of Discovery: How Artificial Intelligence has
Revolutionized the Biotechnology. Nepal Journal of Biotechnology.
2024;12(1):1–11.
93. Park JS, Suryaprakash S, Lao YH, Leong KW.
Engineering mesenchymal stem cells for regenerative medicine and drug delivery.
Methods. 2015;84:3–16.
94. Zhang C, Yan L, Wang X, Zhu S, Chen C, Gu Z,
et al. Progress, challenges, and future of nanomedicine. Nano Today.
2020;35:101008.