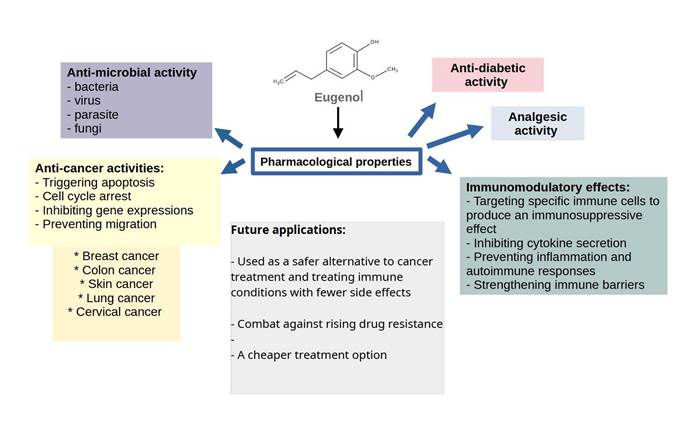
Introduction
Cancer
is a complex disease that effects millions of people around the world. It
results from mutations occurring in genes that control the overall functions of
the cell. These genetic mutations can disrupt the normal cellular metabolism,
especially in the cell cycle, leading to uncontrollable proliferation,
resulting in a tumor (1). It was estimated that in 2020 there were 19.3 million
new cases of cancer and 10 million deaths related to the disease (2). During
2015-2019, it was reported that incidence rates have increased by 0.6-3% for
breast, kidney, liver and cervical cancers in the United States (3). This
alarming rise in cases warrants the need for more effective treatment methods.
Conventional methods typically include chemotherapy, radiotherapy and surgery.
However these approaches face problems that effect the overall efficiency of
the treatment such as serious adverse effects and development of drug
resistance (4). Therefore new therapeutic approaches are being studied and
developed to treat cancer in a more effective approach. These methods include
targeted therapy, stem cell therapy, nanoparticles, chemodynamic therapy and
many more (5). The immune system is also said to play important roles that can
either promote growth of cancer through factors such as inflammation or target
specific cancer cells through targeted therapy.
In
the recent years, many therapeutic options now include alternative and herbal
medicine due to benefits such as fewer side effects and cheaper cost (6). The
use of medicinal plants in healthcare has been an ongoing practice for
thousands of years in many societies. Such plants were known to possess various
medicinal properties that were utilized to treat diseases such as diabetes,
inflammatory conditions and cancer. These pharmacological properties are
contributed by secondary plant metabolites called phytochemicals. These
compounds can be extracted and modified for drug formulations in the
pharmaceutical sector, broadening options available for treating diseases.
The
clove (Syzygium aromaticum) is an excellent example of a herbal species
used for its vast properties. It has been shown to be rich in phytochemicals
such as flavonoids, polyphenols, hidroxibenzoic acids and more (7). These
compounds contribute to numerous bioactive properties that have been used in
traditional medicine, such as antimicrobial properties, anti-inflammatory,
analgesic, anti-cancer activities and many more (8). Eugenol, a major compound
present in clove, has been shown to be effective in inhibiting cancer growth in
various cell lines. In addition, research has shown that eugenol possess
anti-inflammatory activities that can be used to treat inflammatory conditions.
This paper presents the current findings on the anti-cancer effects and immunomodulatory
actions of the compound eugenol, and evaluates its potential in treating cancer
and immune related conditions.
1.
The link between cancer and immune activity
The
immune system is a defensive system that protects the body against foreign
substances that could potentially cause harm. In addition to eliminating
infection, it is involved in many other physiological processes such as the
development, repair and healing of wounds, thus maintaining tissue homeostasis
and integrity (9). The first defense
mechanisms involve the innate immune responses, which comprises of white bloods
cells such as neutrophils and macrophages that engulf and destroy the foreign
substance in a process known as phagocytosis. This first line of defense also
involves non-hematopioetic components such as the epithelial linings of the
respiratory and gastrointestinal tracts, that rid of the infection through
mechanical actions such as mucusal linings (10). If the infection is not
resolved, dendritic cells will stimulate he second line of defense, the
adaptive immune response, which is long lasting and more specific than the
innate immune system (11). It comprises of lymphocytes, such as T helper cells
and B cells, that function to produce antibodies leading to immunity against
the infecting pathogen. These groups of immune cells, including cytotoxic T
cells and natural killer (NK) cells, play an important role in targeting and
eliminating cancer cells (12). Some the functions include triggering apoptosis,
engulfment and immune cell recruitment and more (13). These responses are
triggered by humoural components known as cytokines, which also trigger
inflammation. Inflammation is characterized by swelling, redness and pain that
removes the infectious substance and initiates the healing process (14).
However, inflammation, specifically chronic inflammation, is also the sole
source of any problems that involve inflammation mediated tissue injuries,
which can occur in organs such as the heart, lungs, brain and other systems,
leading to cardiovascular disease, diabetes and cancer (15).
It
has been shown that oxidative stress, cancer and chronic inflammation are
closely linked together (16). The reactive oxidative species (ROS) released
during an inflammation process can promote cell mutations and proliferation,
and in turn, the tumor cells may cause the overexpression of pro-inflammatory
mediators that could stimulate immune cells for further cytokine production
(17) (figure 1). This creates an ‘immune dialogue' between cancer and immune
cells. Targeting these interactions could act as a therapeutic option in
treating conditions resulting from these factors.
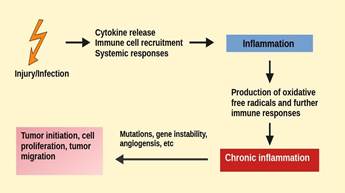
Figure
1.
Tumor formations resulting from immune reactions and inflammation.
2.
Eugenol
Eugenol,
also known as 4-allyl-2-methoxyphenol or eugenic acid, is a natural
phenylpropanoid with an allyl chain-substituted guaiacol found in several
herbal plant species (18). It is the major bioactive compound found in clove,
with concentrations varying between 9 381.70 to 14 650.00 mg per 100 g of the
plant (19). Eugenol can be isolated from plant essential oils using extraction
methods such as steam distillation and microwave assisted extraction, and was
utilized for commercial use by the United States in the 1940s (20). In the
industry, eugenol has been used as a food flavoring and preserver, as well to
treat toothaches and pulpitis, and acts as a flourishing agent, an allergen, a
sensitiser, an anesthetic, a radical scavenger, and many more roles (figure 2)
(21). It has been classified generally recognized as safe (GRAS) chemical by
the World Health Organization (WHO) and is labeled as a nonmutant (22).
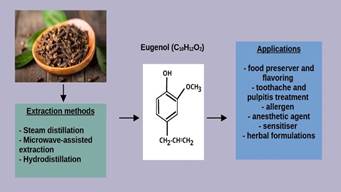
Figure
2.
Extraction methods and applications of eugenol compound.
Some
of the pharmacological properties that eugenol processes includes antimicrobial
activity (anti-bacterial, anti-viral, anti-parasitic and anti-fungal
activities), analgesic activity, and many more (18). Studies have shown that
eugenol also possesses anti-diabetic effects, such as stimulating glucose
uptake and facilitating insulin sensitivity, thus acting as a therapeutic agent
in treating type 2 diabetes (23). Due to its structure possessing hydroxyl
groups, eugenol is able to scavenge toxic free radicals that can cause cell or
tissue damage, leading to mutations (24). Quantitative studies have
demonstrated its strong scavenging effect on DPPH
(2,2-diphenyl-1-picrylhydrazyl) radicals and inhibition on ROS (25). Thus,
eugenol is labeled as a strong antioxidant.
Many
research is available demonstrating the beneficial properties of eugenol in
treating diseases like cancer (26). The polyphenol has been shown to reduce
proliferation and migration in various cancer cell lines by targeting gene
expressions and specific molecular pathways which trigger apoptosis (27). Thus,
making eugenol an ideal candidate in cancer targeted treatments. Eugenol has
also been shown to possess anti-inflammatory properties by reducing the gene
expression of pro-inflammatory cytokines, such as cyclooxygenase 2 (COX-2), and
cascade reactions that regulate the inflammatory process (28). Therefore,
eugenol has potential to used in therapeutic interventions in treating
inflammatory mediated diseases.
3.
Anti-cancer mechanisms of eugenol
Mutations
which trigger oncogenesis can happen in various components of the cell
signaling pathway, such as nuclear proteins, cell surface receptors, kinases,
phosphatases and cytoplasmic enzymes (29). Targeting these specific genetic
mutations and metabolic pathways can lead to the development of targeted
therapies in cancer patients. Eugenol has shown to exert anti-cancer activities
against different cancer cell lines through various mechanisms of action (table
01). Such anti-cancer activities typically include inhibiting cell
proliferation, cell migration, triggering apoptosis and cell cycle arrest, and
many more.
3.1
Breast cancer
Breast
cancer is the most commonly diagnosed malignant cancer in women worldwide.
Despite advances in early detection, mortality rates have not changed (30).
Therefore, new therapeutic methods are currently studied to combat against the
disease by targeting specific signaling pathways that promote proliferation and
malignancy. Such pathways involve proto-oncogenic signaling pathways like Wnt,
Notch, SHH, and many more (31).
Research
has shown that eugenol is effective against breast cancer cells through various
mechanisms of action. A study demonstrated the anti-metastatic effect of
eugenol against MDA-MB-231 and SK-BR-3 breast cancer cell lines, where a
significant reduction in levels of MMP gene expression was observed, indicating
that eugenol may be effective in suppressing triple negative and HER2-positive
breast cancer metastasis (32). Another study revealed that eugenol inhibited
the proliferation of triple-negative breast cancer cells by targeting the
NOD1-NF-κB signaling pathway (33).
An
early study revealed that eugenol at low doses (2uM) showed specific toxicity
against breast cancer cells, mediated by inducing apoptotic pathway and
down-regulation of E2F1/survivin pathway (34). Another study demonstrated that
eugenol was able to trigger apoptosis in melanoma breast cancer cells in vitro
by causing disruptions of the G2/M phase of cell cycle, and mitochondrial
toxicity (35).
3.2
Colon cancer
Colon
cancer is the third most most common cancer diagnosed worldwide and the second
most common cause of mortality due to cancer (36). Various molecular signaling
pathways have been attributed to the development of the cancer. Common
mechanisms include Notch, PI3K/AKT pathway, Wnt, mitogen-activated protein
kinase (MAPK) cascades and more. Mutations in these signaling pathways has been
linked to the progression and development of colon cancer (37).
Many
studies support the idea of using eugenol as a chemoprotective agent against
colon cancer. It has been demonstrated that eugenol was able to reduce the cell
viability of HT-29 colorectal adenocarcinoma cells in a dose-time dependent
manner (38). The same study showed that treatment with IC50 of 500uM
significantly increased the levels of p53 tumor suppressor genes and APC, and a
decrease in KRAS oncogene expression. These genes are generally involved in the
colonrectal cancer progression. An early study showed that eugenol suppressed
the gene expression of COX-2 in HT-29
human colon cancer cells (39).
3.3
Skin cancer
Skin
cancer is the fifth most common cancer diagnosed worldwide, and is expected to
rise in cases in the next 20 years (40). It comes in many types such as
melanoma, basal cell carcinoma and cutaneous squamous cell carcinoma, and a
majority of its cases is caused by exposure to ultraviolet radiation (UV) (41).
Eugenol
has been shown to protect against chemically induced skin cancer by inhibiting
gene expressions of COX-2 and iNOS, as well as signaling molecules such as
NF-kappaB (2). The study also demonstrated an increase in p53 expression and
p21(WAF1) levels in epidermal cells after treatment with eugenol, leading to
apoptosis. An in vivo study showed that treatment with eugenol reduced the size
and incidence of skin tumors at the dysplastic stage in Swiss mice (43). The
experiment showed that eugenol treatment led to the downregulation of c-Myc,
H-ras and Bcl2 expression, whilst upregulating expressions of P53, Bax and
active Caspase-3 in the lesions.
3.4
Lung cancer
Lung
cancer is one of the leading cause of death in men with a poor prognosis (44).
The common frequently altered genes that occur in 35% of lung cancers are RAS
genes that control cell proliferation, NEU gene that is associated with
prognosis, p53 and many others that promote the cell proliferation (45).
It
has been demonstrated that treatment with eugenol in lung cancer ademocarcinoma
A549 cells reduced expressions of phosphate-Akt and MMP-2 activity via PI3K/Akt
pathway, inhibiting its cell proliferation, invasion and migration (26).
Another study showed that in diethylnitrosamine (DENA)/acetylaminofluorene
(AAF) administrated rats, that were then induced with lung cancer, treatment
with eugenol exhibited a significant decrease in BcL-2 expression and an
increase in p53 and Bax expressions (46). This indicates that eugenol possesses
anti-proliferative properties against lung cancer cells.
3.5
Cervical cancer
Cervical
cancer is labeled as the second most common cancer diagnosed in women globally,
that is linked with infection of the human papillomavirus (HPV) (47). Gene
abnormalities that play a role in the pathogenesis of the cancer include c-myc
oncogene, ras genes, cyclin dependent kinases and more (48). Targeting these
genes and treating HPV infection with vaccines are common therapeutic methods
under development for cervical cancer. Eugenol has shown promise in its
anti-cancer effects against cervical cancer cell lines. A study demonstrated
its anti-cancer effects against HeLa cells, showing a decrease in Snail-1 and
vimentin gene expressions, inhibiting cancer migration (49). Another study
showed that treatment with eugenol in HeLa cells resulted in a downregulation
of Bcl-2, COX-2, and IL-1β genes, triggering apoptosis (50).
Table
1.
Summary of anti-cancer mechanisms of eugenol against cancer types.
|
Cancer type
|
Treatment dosage
|
Mechanism of action
|
References
|
|
Breast cancer
|
2uM
|
Downregulation
of the NOD1-NF-κB signaling pathway
and the E2F1/survivin pathway
|
(33,34)
|
|
Colon cancer
|
500uM
|
Reduced expressions of COX-2 and KRAS, and increase p53 gene
expressions
|
(38,39)
|
|
Skin cancer
|
1% eugenol in
acetone
|
Inhibited
expressions of COX-2, c-Myc, H-ras and Bcl2 genes and increased p53 and p21
expressions
|
(42,43)
|
|
Lung cancer
|
1000uM
|
Reduced expressions of MMP-2, BcL-2 and phosphate-Akt activity
whilst increasing p53 and Bax expressions.
|
(26, 46)
|
|
Cervical
cancer
|
200 uM
|
Prevents cell
migration by targeting Snail-1 and
vimentin gene expressions and triggers apoptosis
|
(49, 50)
|
4.
Effects of eugenol on immune system
The
concept of using materials that establish the immune system’s ability to
prevent or treat diseases is referred to as immunotherapy. Its aim is to
balance the immune responses such that it eliminates cancer cells whilst
preventing any autoimmune inflammatory responses (51). This type of therapy is
selective and therefore personalized for each patient.
It
is widely known that eugenol exerts some effects on the immune responses of the
body. Traditionally, it has been used to relieve conditions such as asthma and
allergies. A research study investigating its mechanisms of action revealed
that eugenol is able to halt T cell proliferation and exerted an
immunosuppressive effect on dendritic cells (52). This indicates that eugenol
can be used to control autoimmune and hypersensitivity conditions. A group of
immune cells called myeloid derived suppressor cells (MDSCs), plays a role in
tumor cell progression through their immunosuppressive activity in the tumor
environment (53). Eugenol has a selective inhibitory effect on these cells in a
dose-dependent manner, triggering apoptosis via the intrinsic pathway (54).
Many
research has shown that eugenol possesses significant anti-inflammatory
activities that can be unitized to treat inflammatory related diseases. A study
by Kaur et al demonstrated the anti-inflammatory activity of eugenol in
mice by showing a decrease in proinflammatory cytokines (TNF-alpha, IL-6 and
PGE2) levels, and COX-2, iNOS and ODC activity (42). Another research study
showed that pretreatment with eugenol in procine intestinal epithelial cells
with lipopolysaccharide (LPS) induced inflammation inhibited LPS stimulated
IL-8 levels and the mRNA of TNF-alpha (55). A recent study showed that eugenol
is able to interfere with the NLRP3 inflammasome assembly and IL-1 beta
production, and is involved in post-transcriptional mechanisms that regulated
the inflammation process (56). These findings suggests that eugenol exerts
significant anti-inflammatory actions through different mechanisms of action,
which can be used to treat various conditions related to inflammation. For
example, an interesting study by Lee et al revealed that eugenol was
able to suppress pro-inflammatory mediators as well as immune cell infiltration
into mice spinal cords induced with autoimmune encephalomyelitis. Thus reducing
the symptoms of the autoimmune disease (57). By inhibiting inflammatory
actions, eugenol is able to reduce any cellular or tissue damage exerted by the
mediators, which could contribute to future tumor development (figure 3).
Eugenol
has been shown to effect the immune responses in many other ways as well. For
example, an early study suggested that eugenol has a dose-dependent enhancing
and suppressive effects on the immune response in mice (28). Eugenol has also
been shown to stimulate the production of mucus in mice intestine,
strengthening the mucosal barrier against invading pathogens and diseases (58).
Overall, the effects of eugenol on the immune system has shown to be beneficial
in preventing and treating illnesses.
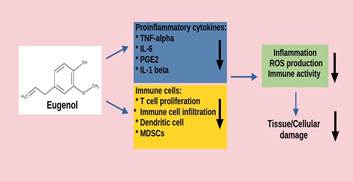
Figure
3.
Eugenol inhibiting inflammation and immune activity leading to less cellular
damage.
5. Enhancing eugenol therapeutic properties
It
is possible through utilizing different methods that the therapeutic properties
of a compound can be enhanced to achieve better results. In the recent years,
the field of nanotechnology has been incorporated into herbal treatment
methods. It involves the use of nanoparticles which are synthesized from
biodegradable lipids, polymers and other safe materials, with the purpose of
developing dosages within the range of 1 to 100nm (59). Polymetric
nanoparticles and solid lipid nanoparticles are common forms nanoparticles used
in research studies with advantages such as increased bioavalibility of drug
and improved physicochemical stability (60). By using nanoparticle drug
delivery systems, the drugs are delivered in a controlled manner to the
targeted area of treatment, which reduces the development of resistance and
adverse effects whilst increasing the efficiency of the drug (61). A study
demonstrated that enzyme-responsive nanoparticles loaded with eugenol was able
to prevent the invasion and migration of colorectal cancer cells at high doses
whilst maintaining a low concentration among the healthy cells (62).
Another
way to enhance the therapeutic properties of a substance is to combine them
with other drugs that will act in a synergized manner. Many studies have shown
that eugenol combined with other chemotherapeutic drugs can enhance its overall
anti-cancer effect (63). Methyl eugenol, when combined with cisplatin, induces
apoptosis, cell cycle arrest and anti-cancer activities against HeLa cervical
cancer cell lines (64). Another study showed that the combination of eugenol
and gemcitabine was able to induce apoptosis and growth inhibition in HeLa
cells at lower concentrations, thus minimizing toxicity in healthy cells (50).
Eugenol is also able to increase the sensitivity of pancreatic cancer cells to
tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) which induces
apoptosis (65).
6.
The need for alternative therapeutic options
One
of the main reasons alternative treatment options are required is due to the
rise in drug resistance. It is mentioned that 90% of failures in chemotherapy,
the most promising cancer treatment, is related to cancer drug resistance in
patients (66). This happens when cancer cells become desensitized towards
common drug treatments by altering the drug targets, activating survival
defense pathways, and other resistance mechanisms that aid the cancer cell in
evading the cytotoxic treatment methods (67). By incorporating different drug
formulations derived from herbal plants and the use of nanotransport systems,
the resistance mechanisms can be bypassed and allow for a better therapeutic
effect with less side effects (61). In addition, the cost of treatment would be
cheaper in comparison to synthetic chemotherapeutic drug usage, allowing it to
be affordable to more patients. Clinical trails are required to test the long
term effects of eugenol on the human body in order to establish the efficacy
and safety of the compound.
Conclusion
Eugenol
has shown great promise in its potential as an anti-cancer drug and its
therapeutic effects against inflammatory conditions. The available research has
showed that the bioactive compound acts by targeting specific molecular
pathways in different cancer types preventing proliferation and cell migration.
Eugenol is also able to effect the immune responses by acting on molecular
components such as cytokines and immune cells. Using eugenol provides many
benefits such as reduced drug resistance development and serious adverse
effects. Therefore, through the utilization of herbal based formulations, more
efficient therapy can be developed in the future against diseases which are
typically difficult to treat due to treatment cost and complications. Further
research must be conducted to pave the way to clinical trails and eventually in
the treatment sector.
Acknowledgments
No
financial support was required for the preparation and publication of this
paper.
Author
contribution
The main manuscript was researched and prepared by
FHH.
Conflict
of interest
The
author declares no conflict of interest associated with this paper.
Funding
There
is no funding.
References
1. Hassanpour SH, Dehghani M. Review of cancer
from perspective of molecular. J Cancer Res Pract. 2017;4(4):127–9.
2. Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN
Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries.
CA Cancer J Clin. 2021;71(3):209–49.
3. Siegel RL, Giaquinto AN, Jemal A. Cancer
statistics, 2024. CA Cancer J Clin. 2024;74(1).
4. Partridge AH, Burstein HJ, Winer EP. Side
Effects of Chemotherapy and Combined Chemohormonal Therapy in Women With
Early-Stage Breast Cancer. JNCI Monographs. 2001;2001(30):135–42.
5. Debela DT, Muzazu SG, Heraro KD, Ndalama
MT, Mesele BW, Haile DC, et al. New Approaches and Procedures for Cancer
treatment: Current Perspectives. SAGE Open Med. 2021;9:205031212110343.
6. Kumar S, Mittal A, Babu D, Mittal A. Herbal
medicines for diabetes management and its secondary complications. Curr
Diabetes Rev. 2020;16(4).
7. Gosavi NS, Koli SS, Jire DS, Shaikh AZ.
Clove (Syzygium Aromaticum): A Miraculous Spice. AJPTR. 2020;8(5):1–17.
8. Vinay Kumar Pandey, Srivastava S, Ashish,
Kshirod Kumar Dash, Singh R, Aamir Hussain Dar, et al. Bioactive properties of
clove (Syzygium aromaticum) essential oil nanoemulsion: A comprehensive review.
Heliyon. 2024;10(1):e22437–7.
9. Sattler S. The Role of the Immune System
Beyond the Fight Against Infection. Adv Exp Med Biol. 2017;1003:3–14.
10. Kaur BP, Secord E. Innate Immunity. Pediatr
Clin North Am. 2019;66(5):905–11.
11. DeMaio A, Mehrotra S, Sambamurti K, Husain S.
The role of the adaptive immune system and T cell dysfunction in
neurodegenerative diseases. J Neuroinflammation. 2022;19(1).
12. Chang RB, Beatty GL. The interplay between
innate and adaptive immunity in cancer shapes the productivity of cancer
immunosurveillance. J Leukoc Biol. 2020;108(1):363–76.
13. Gonzalez H, Hagerling C, Werb Z. Roles of the
Immune System in cancer: from Tumor Initiation to Metastatic Progression. Genes
Dev. 2018;32(19-20):1267–84.
14. Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J,
et al. Inflammatory Responses and inflammation-associated Diseases in Organs.
Oncotarget. 2018;9(6):7204–18.
15. Greten FR, Grivennikov SI. Inflammation and
Cancer: Triggers, Mechanisms, and Consequences. Immunity. 2019;51(1):27–41.
16. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal
BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic
Biol Med. 2010;49(11):1603–16.
17. Djaldetti M. Modulators affecting the immune
dialogue between human immune and colon cancer cells. World J Gastrointest
Oncol. 2014;6(5):129.
18. Nisar MF, Khadim M, Rafiq M, Chen J, Yang Y,
Wan CC. Pharmacological Properties and Health Benefits of Eugenol: A
Comprehensive Review. Oxid Med Cell Longev 2021;2021:1–14.
19. Cortés-Rojas DF, de Souza CRF, Oliveira WP.
Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed.
2014;4(2):90–6.
20. Sellamuthu R. Eugenol. Encyclopedia of
Toxicology. 2014;539–41
21. Pramod K, Ansari SH, Ali J. Eugenol: A
Natural Compound with Versatile Pharmacological Actions. Nat Prod Commun.
2010;5(12):1934578X1000501.
22. Negin Tavvabi-Kashani, Maede Hasanpour, Vafa
Baradaran Rahimi, Naser Vahdati-Mashhadian, Vahid Reza Askari. Pharmacodynamic,
pharmacokinetic, toxicity, and recent advances in Eugenol’s potential benefits
against natural and chemical noxious agents: A mechanistic review. Toxicon.
2024;238:107607–7.
23. Al-Trad B, Alkhateeb H, Alsmadi W, Al-Zoubi
M. Eugenol ameliorates insulin resistance, oxidative stress and inflammation in
high fat-diet/streptozotocin-induced diabetic rat. Life Sci. 2019;216:183–8.
24. Pizzino G, Irrera N, Cucinotta M, Pallio G,
Mannino F, Arcoraci V, et al. Oxidative Stress: Harms and Benefits for Human
Health. Oxid Med Cell Longev. 2017;2017(8416763):1–13.
25. Pérez-Rosés R, Risco E, Vila R, Peñalver P,
Cañigueral S. Biological and Nonbiological Antioxidant Activity of Some
Essential Oils. J Agric Food Chem. 2016;64(23):4716–24.
26. Fangjun L, Zhijia Y. Tumor suppressive roles
of eugenol in human lung cancer cells. Thorac Cancer. 2017;9(1):25–9.
27. Duan Y, Huang X, Qiao B, Ma R, Li J. Eugenol
Inhibits the Biological Activities of an Oral Squamous Cell Carcinoma Cell Line
SCC9 via Targeting MIF. Anti-Cancer Agents Med Chem. 2022;22(15):2799–806.
28. Gojani EG, Wang B, Li DP, Kovalchuk O,
Kovalchuk I. Anti-Inflammatory Properties of Eugenol in
Lipopolysaccharide-Induced Macrophages and Its Role in Preventing β-Cell
Dedifferentiation and Loss Induced by High Glucose-High Lipid Conditions.
Molecules. 2023;28(22):7619.
29. Sinkala M. Mutational landscape of
cancer-driver genes across human cancers. Sci. Rep. 2023;13(1):12742.
30. Smolarz B, Nowak AZ, Romanowicz H. Breast
Cancer—Epidemiology, Classification, Pathogenesis and Treatment (Review of
Literature). Cancers. 2022;14(10):2569.
31. Nwabo Kamdje AH, Seke Etet PF, Vecchio L,
Muller JM, Krampera M, Lukong KE. Signaling pathways in breast cancer:
Therapeutic targeting of the microenvironment. Cell Signal.
2014;26(12):2843–56.
32. Abdullah ML, Hafez MM, Al-Hoshani A,
Al-Shabanah O. Anti-metastatic and anti-proliferative activity of eugenol
against triple negative and HER2 positive breast cancer cells. BMC Complement
Altern Med. 2018;18(1).
33. Shi X, Zhang W, Bao X, Liu X, Yang M, Yin C.
Eugenol modulates the NOD1-NF-κB signaling pathway via targeting NF-κB protein
in triple-negative breast cancer cells. Front Endocrinol. 2023;14.
34. Al-Sharif I, Remmal A, Aboussekhra A. Eugenol
triggers apoptosis in breast cancer cells through E2F1/survivin
down-regulation. BMC Cancer. 2013;13(1).
35. Júnior PL de S, Câmara DAD, Costa AS, Ruiz
JLM, Levy D, Azevedo RA, et al. Apoptotic effect of eugenol envolves G2/M phase
abrogation accompanied by mitochondrial damage and clastogenic effect on cancer
cell in vitro. Phytomedicine. 2016;23(7):725–35.
36. Roshandel G, Ghasemi-Kebria F, Malekzadeh R.
Colorectal Cancer: Epidemiology, Risk Factors, and Prevention. Cancers.
2024;16(8):1530.
37. Koveitypour Z, Panahi F, Vakilian M, Peymani
M, Seyed Forootan F, Nasr Esfahani MH, et al. Signaling pathways involved in
colorectal cancer progression. Cell Biosci. 2019;9(1).
38. Elham Ghodousi-Dehnavi, Hosseini RH, Arjmand
M, Nasri S, Zamani Z. A Metabolomic Investigation of Eugenol on Colorectal
Cancer Cell Line HT-29 by Modifying the Expression of APC, p53, and KRAS Genes.
Evid Based Complement Alternat Med. 2021;2021:1–9.
39. Kim SS, Oh O-Jin, Min HY, Park EJ, Kim Y,
Park HJ, et al. Eugenol suppresses cyclooxygenase-2 expression in
lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Life Sci.
2003;73(3):337–48.
40. Hasan N, Arif Nadaf, Imran M, Umme Jiba,
Sheikh A, Almalki WH, et al. Skin cancer: understanding the journey of
transformation from conventional to advanced treatment approaches. Mol. Cancer.
2023;22(1).
41. Jones OT, Ranmuthu CKI, Hall PN, Funston G,
Walter FM. Recognising Skin Cancer in Primary Care. Adv Ther.
2019;37(1):603–16.
42. Kaur G, Athar M, Alam MS. Eugenol precludes
cutaneous chemical carcinogenesis in mouse by preventing oxidative stress and
inflammation and by inducing apoptosis. Mol Carcinog. 2009;49(3):290–301.
43. Pal D, Banerjee S, Mukherjee S, Roy A,
Chinmay Kumar Panda, Das S. Eugenol restricts DMBA croton oil induced skin
carcinogenesis in mice: Downregulation of c-Myc and H-ras, and activation of
p53 dependent apoptotic pathway. 2010;59(1):31–9.
44. Thakur C. An Overview, Current Challenges of
Drug Resistance, and Targeting Metastasis Associated With Lung Cancer.
2019;21–38.
45. Singh CR, Kathiresan K. Molecular
understanding of lung cancers-A review. Asian Pac J Trop Biomed. 2014;4(Suppl
1):S35–41.
46. Morsy HM, Ahmed OM, Khairy M. A. Zoheir, Adel
Abdel-Moneim. The anticarcinogenic effect of eugenol on lung cancer induced by
diethylnitrosamine/2-acetylaminofluorene in Wistar rats: insight on the
mechanisms of action. Apoptosis. 2023;28(7-8):1184–97.
47. Zhang S, Xu H, Zhang L, Qiao Y. Cervical
cancer: Epidemiology, Risk Factors and Screening. Chin J Cancer Res.
2020;32(6):720–8.
48. Soliman PT, Slomovitz BM, Wolf JK. Mechanisms
of cervical cancer. Drug Discov Today: Dis Mech. 2004;1(2):253–8.
49. Happy Kurnia P, Andika Bachtiar E, Faqrizal
Ria Q, Fadhil F, Anita D. Eugenol isolated from Syzygium aromaticum inhibits
HeLa cancer cell migration by altering epithelial-mesenchymal transition
protein regulators. J Appl Pharm Sci. 2021;
50. Hussain A, Brahmbhatt K, Priyani A, Ahmed M,
Rizvi TA, Sharma C. Eugenol Enhances the Chemotherapeutic Potential of
Gemcitabine and Induces Anticarcinogenic and Anti-inflammatory Activity in
Human Cervical Cancer Cells. Cancer Biother Radiopharm. 2011;26(5):519–27.
51. Abbott M, Ustoyev Y. Cancer and the Immune
System: The History and Background of Immunotherapy. Semin Oncol Nurs.
2019;35(5):150923.
52. Lin CH, Lin SH, Lin CC, Liu YC, Chen CJ, Chu
CL, et al. Inhibitory effect of clove methanolic extract and eugenol on
dendritic cell functions. J Funct Foods. 2016;27:439–47.
53. Van der Veen EL, Bensch F, Glaudemans AWJM,
Lub-de Hooge MN, de Vries EGE. Molecular imaging to enlighten cancer
immunotherapies and underlying involved processes. Cancer Treat Rev.
2018;70:232–44.
54. Ding Y, Yang Z, Zhang W, Xu Y, Wang Y, Hu M,
et al. Eugenol triggers CD11b+Gr1+myeloid-derived suppressor cell apoptosis via
endogenous apoptosis pathway. RSC Adv. 2018;8(7):3833–8.
55. Hui Q, Ammeter E, Liu S, Yang R, Lu P, Lahaye
L, et al. Eugenol attenuates inflammatory response and enhances barrier
function during lipopolysaccharide-induced inflammation in the porcine
intestinal epithelial cells. J Anim Sci. 2020;98(8).
56. Lee J, Hong S, Ahn M, Kim J, Moon C, Matsuda
H, et al. Eugenol alleviates the symptoms of experimental autoimmune
encephalomyelitis in mice by suppressing inflammatory responses. Int
Immunopharmacol. 2024;128:111479–9.
57. Vishteh A, Thomas I, Toshiko Imamura. Eugenol
modulation of the immune response in mice. Immunopharmacol. 1986;12(3):187–92.
58. Wlodarska M, Willing BP, Bravo DM, Finlay BB.
Phytonutrient diet supplementation promotes beneficial Clostridia species and
intestinal mucus secretion resulting in protection against enteric infection.
Sci Rep. 2015;5.
59. Ansari S, Sameem Mohd, Islam F. Influence of
nanotechnology on herbal drugs: A Review. J Adv Pharm Technol. Res.
2012;3(3):142.
60. Silva P, Bonifácio B, Ramos M, Negri K, Maria
Bauab T, Chorilli M. Nanotechnology-based drug delivery systems and herbal
medicines: a review. Int J Nanomedicine. 2013;1.
61. Safhi MM, Sivagurunathan Moni Sivakumar,
Jabeen A, Foziyah Zakir, Islam F, Anwer T, et al. Nanoparticle System for
Anticancer Drug Delivery: Targeting to Overcome Multidrug Resistance. Elsevier
eBooks. 2017;159–69.
62. Nisitha Wijewantha, Sane S, Eikanger M,
Antony RM, Potts RA, Lang L, et al. Enhancing Anti-Tumorigenic Efficacy of
Eugenol in Human Colon Cancer Cells Using Enzyme-Responsive Nanoparticles.
Cancers. 2023;15(4):1145–5.
63. Pal D, Sur S, Roy R, Mandal S, Kumar Panda C.
Epigallocatechin gallate in combination with eugenol or amarogentin shows
synergistic chemotherapeutic potential in cervical cancer cell line. J Cell
Physiol. 2018;234(1):825–36.
64. JL;Shi Y. Myricetin and methyl eugenol
combination enhances the anticancer activity, cell cycle arrest and apoptosis
induction of cis-platin against HeLa cervical cancer cell lines. Int J Clin Exp
Pathol. 2015;8(2).
65. Hyun Hee Kim, Lee SY, Lee DH. Apoptosis of
Pancreatic Cancer Cells after Co-Treatment with Eugenol and Tumor Necrosis
Factor-Related Apoptosis-Inducing Ligand. Cancers. 2024;16(17):3092–2.
66. Mansoori B, Mohammadi A, Davudian S, Shirjang
S, Baradaran B. The Different Mechanisms of Cancer Drug Resistance: a Brief
Review. Adv Pharm Bull. 2017;7(3):339–48.
67. Sameer Ullah Khan, Fatima K, Shariqa Aisha,
Malik F. Unveiling the mechanisms and challenges of cancer drug resistance.
Cell Com Signal. 2024;22(1).