Exploring the role
of the psychedelic alkaloid psilocybin in alleviating cancer-related distress
Fejzić Dino 1 *
1 Bosnalijek JSC, Department of
Research and Registration, Sarajevo, Bosnia and Herzegovina
Corresponding Authors: Fejzić Dino
*
Email: fejzicdino6@gmail.com
Abstract
Cancer is one of the leading causes of
death worldwide, ranking alongside cardiovascular diseases. A cancer diagnosis
is often perceived as a profound and life-altering event, bringing
psychological distress not only to patients but also to their families and
close friends. While advances in cancer treatment have significantly improved
survival rates over the past five decades, progress in addressing the emotional
and psychological burden of the disease has been slower. Oncology patients
require ongoing medical care both in clinical settings and at home, which can
contribute to persistent distress for both patients and their families. Studies
indicate that 20–40% of relatives of cancer patients experience anxiety and depression,
as they must adjust to the shifting realities of serious illness. Given the
limitations of conventional treatments for cancer-related depression, novel
therapeutic approaches are needed. One particularly promising candidate is the
psychedelic alkaloid psilocybin, which has shown potential in multiple
controlled studies. Psilocybin is a naturally occurring compound found in
various species of mushrooms, commonly referred to as psychedelic mushrooms.
Its mind-altering effects have been recognized for centuries, often playing a
central role in religious and spiritual ceremonies. Modern research, however,
has shifted the focus from its historical use to its therapeutic potential.
Research suggests that psilocybin may be highly effective in treating
depression spectrum disorders, especially in cases where traditional treatments
have proven inadequate. If granted regulatory approval, psilocybin could
transform the management of end-of-life depression, offering rapid symptom
relief. Notably, a single dose appears to provide significant and lasting
improvements in depressive symptoms—an essential benefit for cancer patients
with limited life expectancy who require immediate psychological relief.
Keywords: Cancer, Distress, Psilocybin, Depression
Graphical abstract
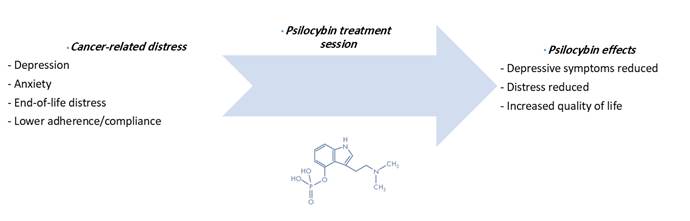
Introduction
Psychological
effects of cancer
While
cancer is not always fatal or even life-threatening, the diagnosis of any type
of cancer brings significant emotional distress to the patient. Many patients
perceive any form of cancer as an immediate death sentence despite many types
of cancer being treatable with ideal outcomes, although certain malignancies
still have a poor outlook, such as pancreatic cancer (1).
In
general, patients can receive either curative treatment when the cancer is
treatable, or they can receive palliative care when the cancer is not
treatable, for example, if the cancer is not operable or does not respond to
chemotherapy or radiation treatments. Palliative care aims to ease suffering as
much as possible and provide the patient with a better quality of life (2).
Depression,
anxiety and end of life distress are often overlooked in both curative
treatments and palliative care. One epidemiological investigation involving
10.153 cancer patients in Canada revealed that 19% of patients had anxiety
symptoms and 12.9% had clinical depression after cancer diagnosis, and another
22,6% of patients had subclinical symptoms (3). Significant differences between
cancer types were also noted where patients with lung, gynecological, or
hematological cancer had the highest level of distress. One interesting insight
into the role of depression and anxiety in cancer comes from public data from
Oregon after the implementation of the Death with Dignity Act. The data shows
that patients who sought prescriptions for lethal drugs were mostly motivated
by non-physical suffering. Statistics show that current or fear of future pain
contributed in 26,4% of the cases, while loss of autonomy (91.4%), decreased
ability to enjoy life (89.7%) and loss of dignity (77.0%) were the most common
reasons for contemplating assisted suicide (4). It is estimated that there are
over 700.000 suicide cases each year among the world’s population, a trend that
is likely to rise (5).
The
management of depression in cancer patients is imperative for both curative
treatment and palliative care. Clinical depression or even depressive symptoms
have a severe negative effect on both short- and long-term life quality and
treatment compliance. Relatives of patients with cancer have a prevalence rate
of depression and anxiety of around 20 – 40 % as the presence of a
life-threatening disease such as cancer requires both the patient and his
immediate family to adapt to new life dynamics (6)
Diagnosing
depression in cancer patients
Depression
is often under-diagnosed in cancer patients and is thus rarely properly
addressed (7, 8). This is mainly because depression is an almost expected
comorbidity of a cancer diagnosis. Establishing a proper diagnosis of
depression in cancer patients is difficult due to overlapping symptoms between
depression and cancer related distress as well as with medication side effects.
Some of the commonly used tools are questionnaires such as HADS (Hospital
Anxiety and Depression Scale), PHQ-9 (Patient Health Questionnaire) and others
(9, 10).
Standardized
questionnaires are often the main tool in psychiatric diagnostics, and while
they are useful tools, they should be considered as a guideline rather than a
gold standard. One important diagnostic criterion in cancer depression is to
determine if the depressive and/or anxiety symptoms were present before the
patient had his first cancer symptoms. This anamnesis should determine whether
the patient had any preexisting depressive or anxiety spectrum disorders prior
to cancer or if the symptoms are completely cancer related. Inspecting the
patient’s history should also determine any findings of prior psychiatric
conditions. if possible, the patient’s relatives should also describe the
patient's character before he or she was diagnosed with cancer or before any
notable symptoms appeared (9, 10, 11).
After
examining the patient’s medical history, a clinical psychologist or
psychiatrist should be included in the care team to assess the scope of any
psychiatric stress and conditions. Another factor to consider is the cancer
itself; if the prognosis is poor, the patient will almost certainly be under
extreme distress or even develop full clinical major depressive disorder (MDD).
Adverse effects from treatments such as hair loss, nausea, vomiting and others
are likely to contribute negatively to further psychiatric development (12).
The
patient should be routinely monitored throughout the treatment and observed for
any signs of depressive and anxiety symptoms (12).
Effects
of depression on cancer morbidity
Multiple
studies have suggested that depression as a comorbidity to cancer leads to a
poorer prognosis and increases the risk of mortality (13).
The
main reasons are a reduction in adherence/compliance and demoralization related
issues:
Reduced treatment adherence and compliance
One common symptom of depression is
hopelessness; the more severe the depression the more severe the feelings of
hopelessness. In some cases, patients simply refuse any treatment, even in
cases where the cancer can effectively be cured. Depressed patients have a
somewhat different viewpoint on their health, often being overly pessimistic or
displaying complete apathy. This can be observed in patients with prior history
of major depressive disorder, especially in its treatment-resistant forms (when
multiple treatment options have failed to provide relief). Reduced adherence
does not necessarily imply complete refusal, sometimes, patients refuse certain
treatments such as chemotherapy or radiotherapy. However, these treatments are
often first line options, and alternative treatments usually have poorer
efficacy. In addition, patients can refuse or forget to take medicine, be less
open to adjuvant treatments, diet regimens, and smoking cessation and be more
prone to substance abuse (14). Thus, if evident depressive symptoms are
present, it is necessary to address them at the beginning of cancer treatment
in order to assure a greater degree of adherence and compliance to primary
treatment.
Demoralization
and suicide ideation
Demoralization
is a common symptom of depression. Patients usually feel that their life has no
meaning or purpose and that they would rather have their life end than continue
to live. This is also the most obvious and prevalent sign of a depressive disorder
and the most common symptom in cancer related distress. Demoralization
significantly lowers the patient’s quality of life and is a major factor in
suicidal behavior. It also affects additional aspects of the patient’s life
such as sleep quality, energy levels, spiritual interests, eating habits and
others (15). Suicide is a major risk; patients with terminal illnesses or those
who experience extreme pain and discomfort are at risk of causing harm to
themselves up to even committing suicide. It is necessary to distinguish
between cancer-related demoralization and depression related demoralization as
patients may experience demoralization without being clinically depressed (16).
A study based on a sample of 8.651.569 patients with cancer, concluded that cancer
patients have a 4-fold increase in completed suicide following a cancer
diagnosis (17). Demoralization and psychological
suffering are often just as impactful as the physical symptoms of cancer. This
existential distress, marked by feelings of hopelessness, loss of purpose, and
fear of death, significantly affects patients' quality of life. It's important
to note that many end-of-life care decisions, including the prescription of
lethal doses, are often made in response to psychological rather than purely
physical reasons. This highlights the necessity of addressing emotional
well-being in cancer patients, as untreated psychological distress can
exacerbate depression and anxiety, leading to a further decline in overall
health (4).
Another
aspect is the effect depression has on terminally ill patients in palliative
care. Functionally, the main goal of palliative care is pain cessation. For
this purpose, a wide variety of pain medications are available to patients,
including morphine, fentanyl, hydrocodone and other opiates, as well as NSAIDs,
tricyclic antidepressants for neurological pain (Amitriptyline) and others.
While pain is often properly addressed, antidepressant and anxiety treatments
are either overlooked or inefficient. Psychosocial treatments and
pharmacotherapy are effective in treating depression in cancer patients.
However, studies have shown limited effectiveness in terminally ill patients
(18). Managing depression is often as important as managing pain when it comes to
palliative care. The absence of depressive and anxiety symptoms will
significantly increase the patient’s quality of life.
The
pharmacological treatment of depression in cancer patients is often complicated
by several factors. The first is drug-drug interactions between antidepressants
and cancer medication. If there are any significant interactions, the priority
in treatment is given to cancer therapy over antidepressants. The second issue
is that antidepressants have significant adverse effects and, most notably, it
takes a minimum of three weeks for them to take effect. This is one of the main
concerns when it comes to standard anti-depressive treatment.
Despite
the widespread use of antidepressants in cancer care, the effectiveness of
antidepressants is still a subject of debate, with some studies showing limited
efficacy (19). Commonly used antidepressants are SSRIs and TCAs with stimulants
being used in some patients such as modafinil or methylphenidate.
In
general, antidepressants are effective and they are first line treatments for
depressive disorders, however, conventional antidepressants still have
significant setbacks. The first issue is that, as mentioned previously, they
need on average 3-4 weeks to have any meaningful effects on the patient’s
condition. This problem is exacerbated when the patient is in significant or
severe suffering or when the patient’s life expectancy is short, for example,
in terminal cancer in its last stage. Even after 3-4 weeks of treatment, we
often do not see a sharp improvement in mood but rather a progressive decrease
in depressive symptoms. One additional downside is that long term treatment is
commonly required which increases the likelihood of significant drug-drug interactions
and adverse effects over time (20, 21).
One
major concern is that a significant portion of patients suffer from what is
called treatment resistant depression, which is also observed in some cancer
patients. There is no definitive definition of treatment resistant depression
(TRD for short), but it is usually considered that a patient has TRD when the
use of two or more antidepressants with different mechanisms of action does not
result in a significant improvement of symptoms (22).
The
first line of treatment for depression spectrum disorders is usually SSRIs. It
is estimated that around 30 – 50% of patients are unresponsive to treatment
(23, 24, 25).
In
conclusion, depression and comorbid anxiety are a significant issue in cancer
patients, reducing both the quality of life and posing a significant obstacle
in curative treatment and palliative care.
An
ideal antidepressant should be administered in a single dose as part of a
treatment plan and provide the patient with a prolonged effect after the drug
is eliminated from the body. A possible candidate for a single dose
antidepressant is the psychoactive alkaloid psilocybin.
Historical
aspects of Psilocybin
Psilocybin
[3-(2-dimethylaminoethyl)-1H-indol-4-yl] dihydrogen phosphate is a tryptamine
molecule that acts as a 5–HT2A receptor agonist. After ingestion,
psilocybin is converted to psilocin (4-hydroxy-N, N-dimethyltryptamine or
4-OH-DMT), meaning that psilocybin is a pro-drug molecule (26).
The
history behind the use of psilocybin is long and detailed. In essence, the use
of psilocybin (although mostly for spiritual purposes) is well documented.
Psilocybin belongs to a group of substances commonly referred to as
hallucinogens, simply meaning that these substances induce hallucinations after
application. This term is somewhat misleading as the effects of these
substances do not lead to typical hallucinations as seen in psychotic
disorders. The use of hallucinogens dates back to ancient times when tribes
used them as part of their spiritual practices (27).
The
majority of these substances today are classified as illegal drugs under most
laws. This is mainly due to the events that took place during the “hippie”
movement in the US when LSD was popularized (along with psilocybin to an
extent). Psilocybin is viewed in the same category as heroin and cocaine from a
legal perspective. However, psilocybin has been extensively researched before
its use was banned and considered as a substance with no medical use. In 1947,
the first trials of LSD were conducted when it was studied as a potential
psychiatric treatment; these trials were largely suspended in 1965. From 1960
to 1970 psilocybin was even marketed under the name INDOCYBINTM, a
pill containing 2 mg of psilocybin (28). In 1970 psilocybin was classified as a
class I substance in the US, which ended any research into its therapeutic
potential.
Psilocybin
attracted a large interest of the scientific community with over 1000 research
papers published between 1950 and the mid-1960s with around 40.000 individuals
taking part in research studies (28, 29). These studies are considered obsolete
by today’s standards, although they do point to a significant interest in the
molecule itself.
The
research into the therapeutic potential of psilocybin and other similar
molecules such as ketamine, LSD and MDMA is again starting to accelerate. For
example, in 2018, Compass Pathways Ltd. received USFDA approval for
“breakthrough therapy” status for psilocybin for treatment resistant
depression. The same year, SPRAVATO® (a ketamine analog) was approved for
treatment resistant depression (Figure 1).
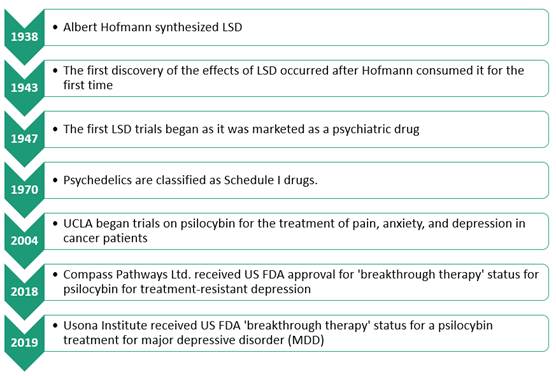
Figure 1. Historical overview of psychedelic compound use and research,
describing key years and events.
Ethical
and legal considerations
Psilocybin
has a significant stigma around its use and application. Most view it as an
illicit substance or street drug that has no place in treatment or a hospital
setting. Many fear potential side effects or long term “insanity” from the use
of psilocybin. While psilocybin has a certain potential for abuse, the same can
be said for many other substances. For example, tramadol has a well described
addictive potential and is commonly abused, the same can be said for any other
opioid. Benzodiazepines and sedatives are one of the most commonly abused drugs
in the world, as are tobacco and alcohol. Using psilocybin to treat end-of-life
distress and severe forms of depression should be viewed the same way as using
opioids to treat severe pain. The ethical and legal
debates surrounding the therapeutic use of psilocybin date back to the 1970s
when it was classified as a Schedule I substance (28). Despite growing evidence
of its potential medical benefits, psilocybin remains a Schedule I drug, meaning
regulatory approval is still required before it can be legally prescribed as a
treatment.
Psilocybin
has one important property that distinguishes it from other addictive
substances: it produces rapid tolerance, meaning that taking one dose after
another will produce little effect (30, 31). This significantly reduces its
abuse potential. Some substances that are completely legal in most countries,
such as nicotine or alcohol, have a greater abuse potential than psilocybin.
For example, nicotine meets the criteria for a schedule III drug according to
CSA (Controlled Substances Act) guidelines. If approved, psilocybin should be
classified as a class IV drug (32).
Psilocybin
should not be considered as a first-line treatment in depression due to the
specifics in its application and the general lack of large clinical studies.
Its use in terminally ill patients and patients with TRD depression is
justified. In addition, access to psilocybin should be monitored and restricted
to medical institutions only.
Pharmacology
Experimental
doses of psilocybin range from 1 to 30 mg, experimental regimens used either a
fixed dose of around 25 – 30 mg or they used body weight-based dosing of
0,2-0,4 mg/kg. One systemic review has shown that a dose of 30 mg / 70 kg
achieves the best results (33).
In a
clinical setting, a well-formulated form of psilocybin in the form of capsules
or tablets should be used. The use of mushrooms containing psilocybin should be
avoided due to varying concentrations of psilocybin as well as the presence of
other active molecules such as muscarine or baeocystin (34). The variation in
content between mushroom species and depending on cultivation factors is often
significant (35, 36).
The
effects of psilocybin start approximately 10 to 40 minutes after ingestion and
usually last between 2 to 6 hours, depending on the dose (37). After ingestion,
psilocybin is metabolized into its active form, psilocin by dephosphorylation
in the intestinal mucosa (38, 39). Psilocin is extensively distributed through
the bloodstream to all tissues and displays linear pharmacokinetics. The
maximum plasma concentration of psilocin after a 25 mg oral dose is 20 ng/mL
120 minutes after ingestion (40). Psilocybin
is eliminated through the kidneys (41) (Figure 2).
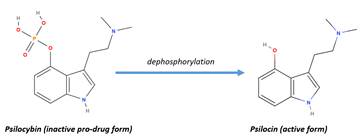
Figure 2. Psilocybin is activated through dephosphorylation to its active form,
psilocin. This means that psilocybin is actually a pro-drug form of psilocin.
The
exact mechanism of action is still undetermined. What is known for certain is
that psilocybin interacts with serotonergic neurotransmission, where, upon
administration, it induces 5-HT2A receptor downregulation (42, 43).
The
fundamental difference between psilocybin and other antidepressants can be
hypothesized on the aspect of neuroplasticity. Neuroplasticity is the brain's
ability to modify and change neuronal connections; in other words,
neuroplasticity is the change capacity of the brain. It is a real possibility
that antidepressants lead to neuroplastic changes in the brain, which would
explain that the majority of antidepressants (ketamine being one exception)
require three or more weeks to take effect (44, 45). Neuroplastic effects have
been demonstrated in animal models (46, 47, 48). Psilocybin seems to have an
immediate effect on the brain’s neuroplasticity. It has been shown that the
therapeutic effects of psilocybin are present after it has been eliminated from
the body, which points to persistent changes in the brain. Still, the effects
of psilocybin are numerous and a definitive mechanism of action is still not
fully understood, which can also be said for many other substances, SSRIs being
one example (49).
Safety
profile
In
comparison to similar substances, psilocybin is considered to have the best
safety profile. The LD50 in rats (intravenous route) for psilocybin
has been determined at 280 mg/kg, which is largely above the therapeutic dose
in humans (50).
The
most frequent somatic adverse reactions observed in trials were nausea,
vomiting and headaches (51). In terms of psychologically adverse reactions, the
most commonly observed were transient anxiety, paranoia, confusion,
derealization, depersonalization and dissociation (51, 52, 53).
Psilocybin
has a favorable safety profile; the main safety issue (as with other similar
substances) is the risk of accidental self-injury (54). Severe psychiatric
adverse reactions with psilocybin have not been reported in clinical studies in
the last 30 years (49, 55).
One
aspect of treatment that is especially important in treating oncology patients
with psilocybin is drug-drug interactions. It is expected that drug-drug
interactions should not be a major issue as psilocybin is only administered in
a single session. That is, the entire treatment consists of a single 25 – 30 mg
dose, after which the drug is eliminated from the body. Interactions with other
medications have not been extensively studied as patients were usually required
to do a full flush-out of any previous medication prior to treatment to prevent
any interactions. Currently described interactions with psilocybin are shown in
table 1.
Table 1. Known drug-drug interactions with psilocybin.
|
Medication
|
Suspected interaction
with psilocybin
|
|
SSRIs
|
Theoretical possibility of inducing serotonin syndrome
The possibility of lowering psilocybin efficacy due to 5-HT2A
downregulation (56)
|
|
Antipsychotics/5-HT2A antagonists
|
Completely block the effects of
psilocybin
|
|
Lithium
|
High risk of seizures (57)
|
|
MAOi
|
Potentiate the effects of psilocybin
(58)
|
|
Caffeine
|
May increase blood pressure and increase undertones of stimulation
(58)
|
|
Cannabis
|
May induce anxiety (58)
|
|
Amphetamines
|
May induce a thought loop*
|
* A
condition where the patient is trapped in a sequence of ideas or thoughts
Interactions
with herbal supplements, food and other medicines are likely but poorly
documented.
Based
on the findings in Table 1, antipsychotics and 5-HT2A antagonists should not be
used concomitantly with psilocybin, as they block its effects, potentially
leading to a lack of treatment efficacy. There is insufficient evidence
regarding the interaction of SSRIs and MAOIs with psilocybin. At this stage,
these drugs should be avoided during psilocybin treatment due to the risks of
severe adverse reactions or potentiation of psilocybin's effects (56, 58).
Lithium should also be avoided due to its high risk of inducing seizures (57).
Additionally, patients should refrain from using any illicit substances,
including amphetamines and cannabis, as their effects may be unpredictable when
combined with psilocybin (58). Currently, the best practice is to introduce a
washout period before initiating psilocybin treatment, as drug-drug
interactions remain insufficiently documented.
Efficacy
of psilocybin – current findings
Modern
psilocybin research is still in its early stages. while there were many papers
published in the last century, most of this data lacks modern clinical
protocols or standards. Thankfully, research has regained momentum in recent
years and there are documented clinical studies involving psilocybin. These
trials focused either on patients with TRD or cancer patients, thus there is at
least some documented evidence in cancer patient treatments involving
psilocybin.
One
case study gives a detailed description of a 54-year-old female patient with
stage IV small cell lung cancer who was on palliative care and had severe
anxiety and depression (59).
The
patient was anxious about her impending death, with feelings of powerlessness
and questioned the meaning of her life. Previous treatment included
escitalopram and sertraline, as well as counseling, which did not prove
beneficial. The patient was subjected to psilocybin treatment where she
received a 5 g dose of psilocybin containing mushrooms (Psilocybe cubensis) and was laid down with eye shades and
headphones with gentle music. The effects of psilocybin wore off after 4-5
hours. Follow-up sessions were conducted the following morning, 1 week and 1
month after the treatment. She completed validated questionnaires (General
Anxiety Disorder–7 questionnaire, Patient Health Questionnaire–9, and McGill
Quality of Life Questionnaire–Revised) that showed marked improvement in her
mood, anxiety, and quality of life, including psychological, existential, and
social subscales. The patient had immediate and sustained improvement in her
psychological and existential distress and an increase in her overall life
quality, describing the experience as the single most personally meaningful
experience in her life.
While
the above article was only a case study with one patient, it provides a
relatively detailed description of the treatment and its benefits. Multiple
trials were conducted in a similar fashion involving anywhere from a few dozen
to a few hundred patients. The Goodwin team conducted the largest psilocybin
trial with 233 participants divided into three groups who had been given a
single dose of psilocybin (1 mg control, 10 mg and 25 mg) (60). While the study
involved patients who suffered from TRD, it can be expected that cancer
patients will show similar results.
There
is also a considerable scientific focus on cancer patients, with multiple
studies demonstrating the efficacy of psilocybin in this treatment group.
Some
of the larger studies and their results involving cancer patients are given in
Table 2.
Table 2. Clinical studies referencing psilocybin treatment in cancer patients.
|
Study
reference
|
n
|
Procedures
|
Duration
|
Outcomes
|
|
Grob et al., 2011 (61)
|
12
|
Randomized controlled trial with a
crossover design of patients with advanced cancer and anxiety who received
two treatment sessions several weeks apart and were blinded to placebo
(niacin 250 mg vs. psilocybin 0.2 mg/kg).
|
Six-hour drug dosing sessions spaced
several weeks apart with self-reported outcomes up to 6 months post-second
session
|
The study showed sustained reduction
in anxiety at one- and three-months post treatment as well as general mood
improvements at six-month follow up.
|
|
Griffiths et al., 2016 (62)
|
51
|
Randomized controlled double-blind
trial of patients with life-threatening cancer-related depression and anxiety
who received psilocybin low/placebo-like dose (1 or 3 mg/70 kg) versus a high
dose (22 or 30 mg/70 kg) administered in counterbalanced sequence.
|
Five weeks between sessions and a
six-month follow-up
|
High dose psilocybin produced
significant drops in death anxiety with an increase in life quality. Changes
were sustained at the six-month follow-up, with approximately 80% of
participants continuing to show significant decreases in depressed mood and
anxiety.
|
|
Ross et al., 2016 (63)
|
29
|
Randomized controlled trial with
crossover design of patients with life-threatening cancer related anxiety and
depression who received single dose niacin versus psilocybin (0.3 mg/kg) in
conjunction with psychotherapy
|
Seven weeks and six and a half months
follow-up
|
A single dose of psilocybin produced
acute and lasting reductions in anxiety and depression as well as benefits in
existential distress.
|
|
Agin-Liebes et al., 2020 (64)
|
15
|
Long-term patient follow-up study of
15 out of 29 willing and surviving participants of the 2016 Ross parent study
to assess previous study efficacy
|
An average of 3.2 to 4.5 years
following the initial administration
|
Anxiety, depression, hopelessness,
demoralization, and death anxiety reductions were sustained at both long-term
follow-ups.
|
|
Agrawal et al., 2023 (65)
|
30
|
Non-randomized controlled trial of
patients with cancer and major depression disorder who received psilocybin 25
mg to create a scalable, rapidly effective treatment in a setting of 1:1
therapist: patient ratio in groups of 3–4
|
Eight weeks
|
Long-term reduction in depressive
symptoms over eight weeks
|
|
Lewis et al., 2023 (66)
|
12
|
Pilot study of psilocybin enhanced
group psychotherapy in patients with cancer in cohorts of four patients who
received three group preparatory sessions, one drug dosing session with
psilocybin 25 mg, followed by three group integration sessions over three
weeks
|
Preparation and drug dosing session
over three weeks, followed by three weeks of integration sessions, outcomes
over six six-month period
|
Significant reduction of depression on
the two and 26-week time points.
|
The studies outlined in Table 2 were
specifically designed for cancer patients rather than individuals experiencing
depression unrelated to cancer. One major limitation is the small sample sizes,
with the largest study, conducted by Griffiths et al., including only 51
participants (62). This highlights the need for larger studies involving
hundreds of participants to generate more reliable data. However, despite their
limited scale, these studies have consistently demonstrated that psilocybin
shows significant potential in treating cancer-related depression, justifying
further research on a larger scale.
A follow-up study by Agin-Liebes et
al. confirmed that the positive effects of psilocybin were long-lasting (64).
Additional research is needed to compare psilocybin with currently approved
antidepressants and to conduct safety studies assessing potential drug-drug
interactions.
The studies in Table 2 were generally
well-designed, but one significant challenge in psilocybin research is the
difficulty of maintaining blinding. While placebo controls were used, the
profound psychoactive effects of psilocybin make it apparent to participants
whether they received the active drug or a placebo. The study by Griffiths et
al. attempted to address this by using a low dose of psilocybin as a control,
but even in this case, participants could likely distinguish between the full
and reduced doses. Given these challenges, future studies may need to be
conducted as open-label trials rather than attempting traditional
placebo-controlled designs.
Conducting
the treatment session – points to consider
The
main benefit of psilocybin treatment is its rapid onset; patients often
experience a reduction of depressive symptoms immediately after the first dose
or first session. One downside of psilocybin is that it requires a specific way
of administration. As a “hallucinogen” or “psychedelic” substance, psilocybin
completely alters the patient’s perception of reality. It is still unclear if
this effect is necessary for antidepressant treatment, however, numerous
patients have described the psychedelic experience as beneficial.
The
psychedelic episode needs to be closely monitored by at least two medical
professionals (preferably psychiatrists) until the effects have subsided and
the patient is fully conscious. There is a significant risk of self-injury if
the patient is left unattended during this treatment.
The
first step to psilocybin treatment is considering if the patient is a candidate
for psilocybin treatment. While there is no definitive guideline as of yet,
there are a few key points to consider:
1)
Assess
the level of distress, anxiety and depression – this step should be complemented
by using an appropriate questionnaire. Priority should be given to currently
approved treatments (SSRIs, benzodiazepines, MAOi) if the symptoms are not
severe. A certain amount of anxiety and/or depression is expected after a
cancer diagnosis.
2)
Consider
the patient’s medical history – if the patient has any history or an active psychotic disorder
such as schizophrenia, this should be considered as an absolute
contraindication for psilocybin treatment (67). Also consider any somatic
issues such as heart arrhythmia, vascular disorders, previous stroke, age or
similar.
If
the patient has developed severe depression, demoralization, and anxiety, and
if the quality of the patient’s life is low due to cancer related distress
(both in terminal and non-terminal patients) and there are no proven
contraindications, consider psilocybin treatment (Figure 3).
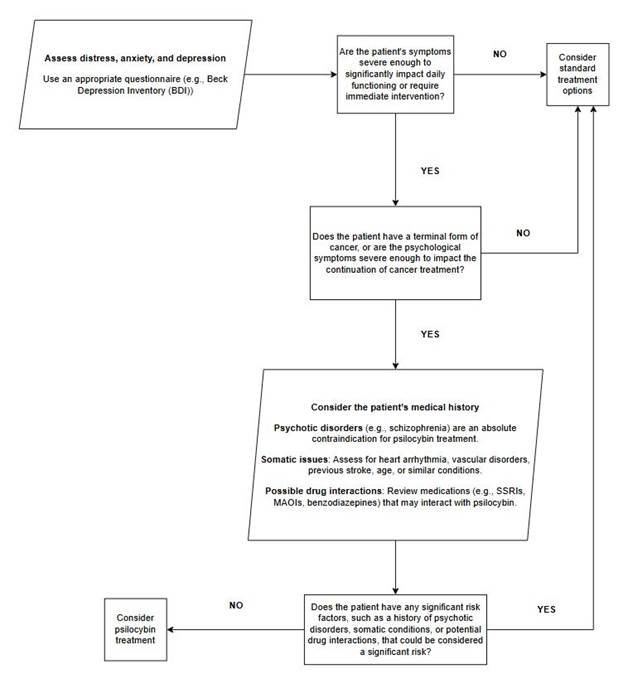
Figure 3. Proposed protocol for evaluating candidates for psilocybin treatment.
Once selected for treatment, the patient should be
given a detailed explanation of what the treatment will look like. Psilocybin
has a distinct effect on the patient’s consciousness and perception of reality.
Unless the patient knows what to expect, these effects can be frightening. A
preparatory session with a trained psychiatrist is recommended (Figure 4).
1)
Any drugs
and medications that are considered contraindicated and carry the possibility
of drug-drug interactions should be temporarily discontinued prior to
treatment.
2)
On the day
of treatment, the patient should be placed in a well-lit room with a calming
atmosphere; gentle music can be played if the patient desires. The ambiance and
surroundings should be welcoming.
3)
Prior to
and during the treatment, the patient’s vital signs should be closely monitored
4)
A 25 mg –
30 mg dose of oral psilocybin should be given to the patient with a full glass
of water.
5)
Reassure
the patient and monitor him until the effects of the drug have worn off
(usually about 6 hours).
6)
Evaluate
the patient post treatment.
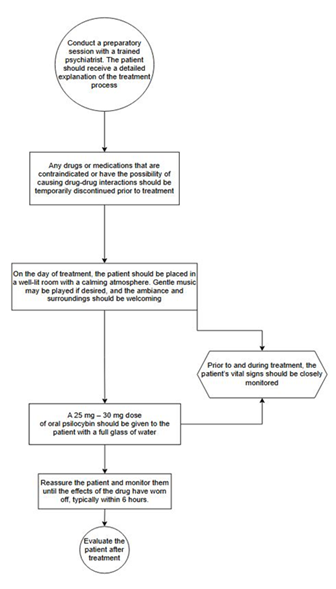
Figure 4. Proposed psilocybin treatment protocol.
If
the patient exhibits any signs of prolonged violent/frightened behavior and is
unresponsive to verbal reassurance and/or there are any severe somatic adverse
reactions, ketanserin blocks most of the effects of psilocybin, making it
possible to cease treatment in case of an emergency (68).
Conclusion
Psilocybin
shows promise in treating depression in cancer patients. Multiple clinical
trials have been conducted in this exact patient group and many others have
been undertaken in clinically depressed patients who do not suffer from cancer.
The main advantage of psilocybin over currently available antidepressants is
its immediate effect, the requirement for only one dose and its rapid effect
onset. If properly conducted, psilocybin treatment has great potential to
become an important component both in palliative care and in antidepressant
treatment in cancer patients. Modern studies that are currently published have
not shown any serious adverse effects, most of these studies involved small
patient groups and larger studies are thus needed to confirm the safety and
efficacy of psilocybin. Based on current findings, it can be concluded that
psilocybin is a viable antidepressant and its use should be considered in
certain cases. Further research with larger clinical
trials is needed to confirm the safety and efficacy of psilocybin. Future
studies should focus on evaluating its long-term effects and overall safety.
Additionally, research on potential interactions with commonly prescribed
medications is essential, as patients in clinical settings often receive multiple
concurrent treatments.
Regulatory approval of this treatment is crucial. If psilocybin
receives the necessary authorization, it will transition from an experimental
compound to a clinically validated and regulated treatment. However, if
approved, its use should be strictly limited to professional administration
within a controlled clinical setting.
Author
contribution
DF fully wrote and edited the manuscript.
Conflict
of interest
The
author declares no conflict of interest.
Funding
There
is no funding.
References
1. McCaffery K, Barratt A, Irwig L.
Perceptions of cancer as a death sentence: prevalence and consequences.
Psychooncology. 2013;22(10):2296-301.
2. Davies E, Gray A. The role of palliative
care in cancer treatment. Eur J Cancer. 2008;44(6):774-9.
3. Linden W, Vodermaier A, Mackenzie R, Greig
D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer
type, gender, and age. J Affect Disord. 2012;141(2-3):343-51.
4. Byock I. Taking psychedelics seriously. J
Palliat Med. 2018 Apr;21(4):417-21. doi:10.1089/jpm.2017.0684.
5. Rădulescu I, Drăgoi AM, Trifu SC, Cristea
MB. Neuroplasticity and depression: rewiring the brain's networks through
pharmacological therapy (Review). Exp Ther Med. 2021;22(4):1131.
6. Friðriksdóttir N, Sævarsdóttir Þ,
Halfdánardóttir SÍ, Jónsdóttir A, Magnúsdóttir H, Ólafsdóttir KL, et al. Family
members of cancer patients: needs, quality of life and symptoms of anxiety and
depression. Acta Oncol. 2011;50(2):252-8.
7. Walker J, Hansen CH, Martin P, Symeonides
S, Ramessur R, Murray G, et al. Prevalence, associations, and adequacy of
treatment of major depression in patients with cancer: a cross-sectional
analysis of routinely collected clinical data. Lancet Psychiatry.
2014;1(5):343-50.
8. Massie MJ. Prevalence of depression in
patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57-71.
doi:10.1093/jncimonographs/lgh014.
9. Vodermaier A, Linden W, Siu C. Screening
for emotional distress in cancer patients: a systematic review of assessment
instruments. J Natl Cancer Inst. 2009;101(21):1464-88.
10. Chhabria KS, Carnaby GD. Psychometric
validation of the Center for Epidemiological Studies-Depression Scale in Head
and Neck Cancer patients. Oral Oncol. 2017;75:158-62.
11. Mitchell AJ, Lord K, Symonds P. Which
symptoms are indicative of DSM-IV depression in cancer settings? J Affect
Disord. 2012;138(1-2):137-48.
12. Donovan KA, Grassi L, Deshields TL, Corbett
C, Riba MB. Advancing the science of distress screening and management in
cancer care. Epidemiol Psychiatr Sci. 2020;29.
13. Pinquart M, Duberstein PR. Depression and
cancer mortality: a meta-analysis. Psychol Med. 2010;40(12):1797-810.
14. Spiegel D, Giese-Davis J. Depression and
cancer: mechanisms and disease progression. Biol Psychiatry.
2003;54(3):269-282.
15. Chang T, Hung C, Huang P, Hsu C, Yen T.
Demoralization and its association with quality of life, sleep quality,
spiritual interests, and suicide risk in breast cancer inpatients: A
cross-sectional study. Int J Environ Res Public Health. 2022;19(19):12815.
16. Fang CK, Chang MC, Chen PJ, Lin CC, Chen GS,
Lin J, Hsieh RK, Chang YF, Chen HW, Wu CL, et al. A correlational study of
suicidal ideation with psychological distress, depression, and demoralization
in patients with cancer. Support Care Cancer. 2014;22(12):3165-74.
17. Zaorsky NG, Zhang Y, Tuanquin L, Bluethmann
SM, Park HS, Chinchilli V. Suicide among cancer patients. Nat Commun.
2019;10(1):207.
18. Williams S, Dale J. The effectiveness of
treatment for depression/depressive symptoms in adults with cancer: a
systematic review. Br J Cancer. 2006;94(3):372-90.
19. Schmauss C. An HDAC-dependent epigenetic
mechanism that enhances the efficacy of the antidepressant drug fluoxetine. Sci
Rep. 2015;5:8171.
20. Fava M, Davidson KG. Definition and
epidemiology of treatment-resistant depression. Psychiatr Clin North Am.
1996;19(2):179-200.
21. Cipriani A, et al. Antidepressants versus
placebo for depression in children and adolescents. Lancet.
2018;391(10115):1331-41.
22. Lazur J, Hnatyk K, Kała K, et al. Discovering
the potential mechanisms of medicinal mushrooms antidepressant activity: a
review. Antioxidants. 2023;12(3):623.
23. Gukasyan N, Davis AK, Barrett FS, et al.
Efficacy and safety of psilocybin-assisted treatment for major depressive
disorder: prospective 12-month follow-up. J Psychopharmacol. 2022
Feb;36(2):151-8.
24. Ziff S, Stern B, Lewis G, et al. Analysis of
psilocybin-assisted therapy in medicine: a narrative review. Cureus.
2022;14(2).
25. Daws RE, Timmermann C, Giribaldi B, et al.
Increased global integration in the brain after psilocybin therapy for
depression. Nat Med. 2022;28:844-851.
26. Thaoboonruang N, Lohitnavy M, Lohitnavy O.
Pharmacokinetics of psilocybin, a tryptamine alkaloid in magic mushroom
(Psilocybe cubensis): a systematic review. J Psychoactive Drugs. Published
online September 10, 2024.
27. Doblin RE, Christiansen M, Jerome L, Burge B.
The past and future of psychedelic science: an introduction to this issue. J
Psychoactive Drugs. 2019;51(2):93-97.
28. Lowe H, Toyang N, Steele B, et al. The
therapeutic potential of psilocybin. Molecules. 2021;26(10):2948.
29. Johnson MW, Griffiths RR. Potential
therapeutic effects of psilocybin. Neurotherapeutics. 2017 Jul;14(3):734-40.
30. Psiuk D, Nowak E, Cholewa K, et al. The
potential role of serotonergic hallucinogens in depression treatment. Life.
2021;11(8):765.
31. Dawood Hristova JJ, Pérez-Jover V.
Psychotherapy with psilocybin for depression: systematic review. Behav Sci.
2023;13(4):297.
32. Johnson MW, Griffiths RR, Hendricks PS,
Henningfield JE. The abuse potential of medical psilocybin according to the 8
factors of the Controlled Substances Act. Neuropharmacology. 2018
Nov;142:143-66.
33. IsHak WW, Garcia P, Pearl R, et al. The
impact of psilocybin on patients experiencing psychiatric symptoms: a
systematic review of randomized clinical trials. Innov Clin Neurosci.
2023;20(4-6):39-48.
34. Wurst M, Kysilka R, Flieger M. Psychoactive
tryptamines from basidiomycetes. Folia Microbiol. 2002;47:3-27.
35. Bradshaw AJ, Backman TA, Ramírez-Cruz V, et
al. DNA authentication and chemical analysis of Psilocybe mushrooms reveal
widespread misdeterminations in fungaria and inconsistencies in metabolites.
Appl Environ Microbiol. 2022;88(24).
36. MacCallum CA, Lo LA, Pistawka CA, Deol JK.
Therapeutic use of psilocybin: practical considerations for dosing and
administration. Front Psychiatry. 2022;13:1040217.
37. Passie T, Seifert J, Schneider U, Emrich HM.
The pharmacology of psilocybin. Addiction Biol. 2002;7(4):357-64.
38. Castro Santos H, Gama Marques J. What is the
clinical evidence on psilocybin for the treatment of psychiatric disorders? A
systematic review. Porto Biomed J. 2021 Feb 11;6(1).
39. Thomann J, Kolaczynska KE, Stoeckmann OV, et
al. In vitro and in vivo metabolism of psilocybin's active metabolite psilocin.
Front Pharmacol. 2024;15:1391689.
40. Heuschkel K, Kuypers KPC. Depression,
mindfulness, and psilocybin: possible complementary effects of mindfulness
meditation and psilocybin in the treatment of depression. A review. Front
Psychiatry. 2020 Mar 31;11:224.
41. Irizarry R, Winczura A, Dimassi O, et al.
Psilocybin as a treatment for psychiatric illness: a meta-analysis. Cureus.
2022 Nov 22;14(11).
42. Hasler F, Grimberg U, Benz MA, et al. Acute
psychological and physiological effects of psilocybin in healthy humans: a
double-blind, placebo-controlled dose-effect study. Psychopharmacology.
2004;172:145-156.
43. Cameron LP, Patel SD, Vargas MV, et al.
5-HT2ARs mediate therapeutic behavioral effects of psychedelic tryptamines. ACS
Chem Neurosci. 2023;14(3):351-358.
44. Levy MJF, Boulle F, Emerit MB, et al. 5-HTT
independent effects of fluoxetine on neuroplasticity. Sci Rep. 2019;9:6311.
45. Thompson SM. Optimizing psychedelic compounds
for neuropsychiatric therapy. Neuropsychopharmacology. 2021;46(8):1397-1398.
46. Cameron LP, Tombari RJ, Lu J, et al. A
non-hallucinogenic psychedelic analogue with therapeutic potential. Nature.
2021;589(7842):474-479.
47. Raval NR, Johansen A, Donovan LL, et al. A
single dose of psilocybin increases synaptic density and decreases 5-HT2A
receptor density in the pig brain. Int J Mol Sci. 2021;22(2):835.
48. Shao LX, Liao C, Gregg I, et al. Psilocybin
induces rapid and persistent growth of dendritic spines in frontal cortex in
vivo. Neuron. 2021;109(16):2535-2544.e4.
49. Benko J, Vranková S. Natural psychoplastogens
as antidepressant agents. Molecules. 2020;25(5):1172.
50. Usdin E, Efron D. Psychotropic drugs and
related compounds. 2nd ed. Bethesda, MD: National Institute of Mental Health;
1972. p. 138.
51. Dos Santos RG, Bouso JC, Rocha JM, et al. The
use of classic hallucinogens/psychedelics in a therapeutic context: healthcare
policy opportunities and challenges. Risk Manag Healthc Policy. 2021;14:901-10.
52. Calderon SN, Bonson KR, Reissig CJ, et al.
Considerations in assessing the abuse potential of psychedelics during drug
development. Neuropharmacology. 2023 Feb 15;224:109352.
53. Yerubandi A, Thomas JE, Bhuiya NMMA,
Harrington C, Villa Zapata L, Caballero J. Acute adverse effects of therapeutic
doses of psilocybin: a systematic review and meta-analysis. JAMA Netw Open.
2024;7(4).
54. Wang SM, Kim S, Choi WS, et al. Current
understanding on psilocybin for major depressive disorder: a review focusing on
clinical trials. Clin Psychopharmacol Neurosci. 2024;22(2):222-231.
55. Zeifman RJ, Singhal N, Breslow L, Weissman
CR. On the relationship between classic psychedelics and suicidality: a
systematic review. ACS Pharmacol Transl Sci. 2021 Mar 11;4(2):436-51.
56. Rosenblat JD, Husain MI, Lee Y, et al. The
Canadian Network for Mood and Anxiety Treatments (CANMAT) Task Force report:
serotonergic psychedelic treatments for major depressive disorder. Can J
Psychiatry. 2023;68(1):5-21.
57. Nayak SM, et al. Classic psychedelic
coadministration with lithium, but not lamotrigine, is associated with
seizures: an analysis of online psychedelic experience reports.
Pharmacopsychiatry. 2021 Aug 4.
58. Vorobyeva N, Kozlova AA. Three
naturally-occurring psychedelics and their significance in the treatment of
mental health disorders. Front Pharmacol. 2022 Jun 28;13:927984.
59. Patchett-Marble R, O'Sullivan S, Tadwalkar S,
Hapke E. Psilocybin mushrooms for psychological and existential distress:
treatment for a patient with palliative lung cancer. Can Fam Physician. 2022
Nov;68(11):823-7.
60. Goodwin GM, Aaronson ST, Alvarez O, et al.
Single-dose psilocybin for a treatment-resistant episode of major depression. N
Engl J Med. 2022;387(18):1637-48.
61. Grob CS, Danforth AL, Chopra GS, et al. Pilot
study of psilocybin treatment for anxiety in patients with advanced-stage
cancer. Arch Gen Psychiatry. 2011;68(1):71-8.
62. Griffiths RR, Johnson MW, Carducci MA, et al.
Psilocybin produces substantial and sustained decreases in depression and
anxiety in patients with life-threatening cancer: a randomized double-blind
trial. J Psychopharmacol. 2016;30(12):1181-97.
63. Ross S, Bossis A, Guss J, et al. Rapid and
sustained symptom reduction following psilocybin treatment for anxiety and
depression in patients with life-threatening cancer: a randomized controlled
trial. J Psychopharmacol. 2016;30(12):1165-80.
64. Agin-Liebes GI, Malone T, Yalch MM, et al.
Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and
existential distress in patients with life-threatening cancer. J
Psychopharmacol. 2020;34(2):155-66.
65. Agrawal M, Emanuel E, Richards B, et al.
Assessment of psilocybin therapy for patients with cancer and major depressive
disorder. JAMA Oncol. 2023;9(6):864-6.
66. Lewis BR, Garland EL, Byrne K, et al. HOPE: a
pilot study of psilocybin enhanced group psychotherapy in patients with cancer.
J Pain Symptom Manage. 2023;66(2):258-69.
67. Halim HJ, Burk BG, Fargason RE, Birur B.
Manic episode following psilocybin use in a man with bipolar II disorder: a
case report. Front Psychiatry. 2023;14:1221131. doi:10.3389/fpsyt.2023.1221131.
Published September 22, 2023.
68. Holze F, Singh N, Liechti ME, D’Souza DC.
Serotonergic psychedelics: a comparative review of efficacy, safety,
pharmacokinetics, and binding profile. Biol Psychiatry Cogn Neurosci
Neuroimaging. 2024;9(5):472-89. doi:10.1016/j.bpsc.2024.01.007.




