Parasite in the
brain: the role of Toxoplasma gondii in brain cancer and
neuropsychiatric disorders
Peyman Rabiei 1,
Mohammad Esmaeilpour-Bandboni 2 *
1 Department of Veterinary Medicine, Babol-Branch, Islamic Azad
University, Babol, Iran
2 Department of Nursing, Zeynab (P.B.U.H) School of Nursing and
Midwifery, Guilan University of Medical Sciences, Rasht, Iran
Corresponding
Authors: Mohammad Esmaeilpour-Bandboni
*
Email: esmaeilmmm@yahoo.com
Abstract
Toxoplasma gondii (T. gondii) is a protozoan parasite that affects about one-third of the world's
human population, frequently creating a dormant presence in the brain. Recent
studies have placed growing emphasis on the possible consequences of T.
gondii infection concerning brain cancer and neuropsychiatric conditions,
such as schizophrenia, bipolar disorder, and depression. This review
consolidates recent discoveries regarding how T. gondii could affect
neurological well-being, especially its capacity to modify neurotransmitter
pathways, adjust immune reactions, and provoke neuroinflammation. We examine
the epidemiological links between T. gondii seropositivity and different
psychiatric disorders, highlighting the necessity for additional research into
the causal mechanisms connecting this parasite to brain pathology. Moreover, we
investigate the possibility of T. gondii as a co-factor in developing
brain tumors, emphasizing its function in immune evasion and modulation of the
tumor microenvironment. Grasping these connections is essential for creating
focused therapeutic approaches and public health measures designed to reduce
the impact of T. gondii infection on mental health and neuro-oncology.
Keywords: Toxoplasma gondii, Brain cancer, Neuropsychiatric disorders, Neurotransmitter
modulation, Neuroinflammation
Introduction
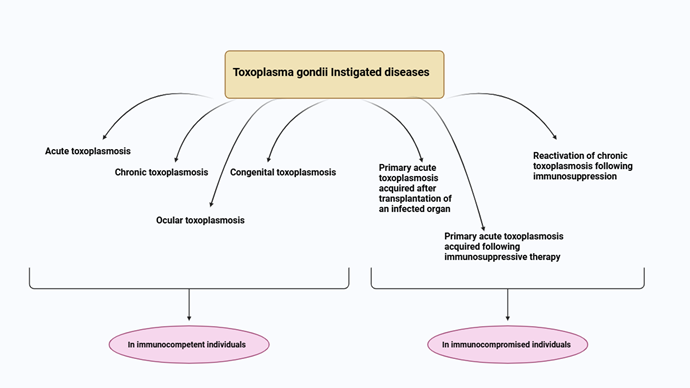
T.
gondii is
an intracellular protozoan parasite that is obligatory and has attracted
considerable attention because of its common occurrence and possible effects on
human health (1). It is estimated that as many as
30% of the worldwide population carries this parasite, frequently without
showing any symptoms. Nonetheless, persistent infections can result in
significant neurological effects, especially if the immune system is weakened
or experiences stress (2). The parasite is mainly spread by
ingesting oocysts found in contaminated food or water, along with vertical
transmission from mother to fetus or via organ transplantation (3). The central nervous system (CNS)
acts as a key reservoir for T. gondii, allowing it to create tissue
cysts that endure for the lifetime of the host (4). These cysts may reactivate when
the immune system is suppressed, resulting in acute toxoplasmosis that can
cause serious neurological symptoms like encephalitis or psychological issues (5). The connection between T.
gondii infection and several neuropsychiatric disorders has been
extensively studied, with research showing a notable link between T. gondii
seropositivity and disorders like schizophrenia, bipolar disorder, and
depression (6).
Mechanisms
of Neuroinvasion
The
processes through which T. gondii enters and influences the brain are
intricate and varied. Once inside the host's body, T. gondii can
traverse the blood-brain barrier (BBB), which is a selectively permeable
barrier that shields the brain from pathogens and permits the passage of vital
nutrients (7). T. gondii's capability to
cross this barrier is linked to its distinctive interactions with host cells
and its ability to influence host immune responses. Upon entering the CNS, T.
gondii can trigger notable alterations in neurotransmitter systems,
especially those related to dopamine and gamma-aminobutyric acid (GABA) (8). Studies have indicated that
infected persons may display changed levels of these neurotransmitters,
essential for mood control and cognitive abilities (9). For example, increased dopamine
levels have been correlated with behavioral alterations seen in both infected
humans and animal models, indicating a possible connection between T. gondii
infection and psychotic disorders like schizophrenia (10) (Figure 1).
Toxoplasma
gondii and Neuropsychiatric Disorders
Toxoplasma
gondii, a
prevalent neurotropic parasite, has become more associated with several
neuropsychiatric disorders in humans. These links encompass schizophrenia,
Alzheimer's disease, and Parkinson's disease, although the precise pathogenic
mechanisms are still not fully understood. T. gondii can remain in the
brain as tissue cysts, requiring an ongoing immune response to stop the
reactivation of the infection (20, 21). Chronic infection is especially
worrying, as evidence indicates it can result in neurodegeneration in certain
areas of the brain, like the anterior cingulate cortex and somatomotor cortex,
impacting both glutamatergic and GABAergic neurons (22, 23). Changes in behavior among infected
individuals may be partially linked to variations in neurotransmitter levels,
especially dopamine. Research has shown that T. gondii infection is
linked to heightened dopamine metabolism, a component associated with the onset
of schizophrenia (20). This connection is additionally
reinforced by evidence indicating that those with T. gondii antibodies
might display elevated rates of aggression, impulsivity, and possibly
heightened risks for suicide and traffic accidents, hinting at wider behavioral
consequences (24, 25). The neuroinflammatory reaction
initiated by T. gondii infection significantly impacts neurobiology,
possibly resulting in alterations in neurotransmitter receptor quantities and
synaptic connections (26). This inflammation may play a role
in the development and progression of multiple neurodegenerative diseases,
since long-term T. gondii infection might encourage neurodegeneration
and neurocognitive irregularities (6, 23, 27). Studies
persist in investigating the intricate connection between T. gondii
infection and neuropsychiatric effects, which affects our comprehension of the
mechanisms behind behavioral alterations and the possibilities for preventive
measures (28).
Conclusion
The
relationship between Toxoplasma gondii (T. gondii) infection and
brain tumors has garnered increasing attention in the scientific community,
particularly regarding its implications for public health and cancer prevention
strategies. This study demonstrates a significant association between T.
gondii infection and various types of brain tumors, including gliomas and
meningiomas. The findings underscore the need for further research to elucidate
this association's underlying mechanisms and explore potential therapeutic
avenues. Recent studies consistently show a higher prevalence of T. gondii
seropositivity among patients with brain tumors compared to healthy
individuals. For instance, a systematic review and meta-analysis identified an
overall odds ratio (OR) of 1.96 for the link between T. gondii infection
and brain tumors, with specific ORs of 1.64 for gliomas and 2.30 for
meningiomas. These findings suggest that individuals exposed to T. gondii
may have approximately double the risk of developing brain tumors, highlighting
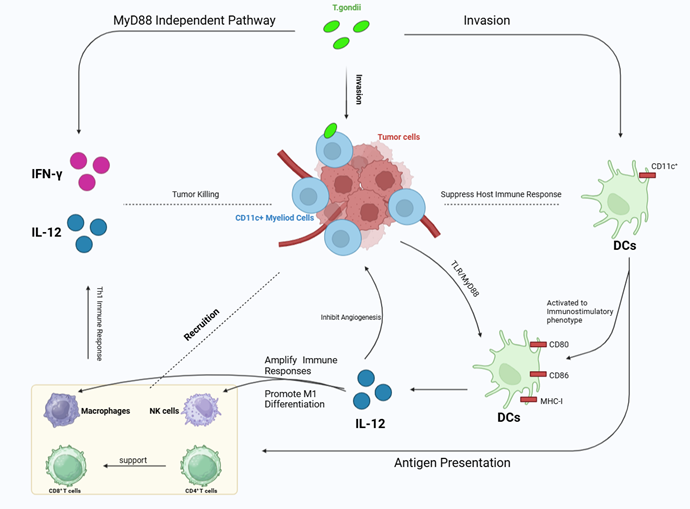
the need for further investigation. One proposed mechanism by which this
parasite may contribute to tumorigenesis is its ability to modulate the tumor
microenvironment. The parasite’s invasion and persistence in the central
nervous system could lead to chronic inflammation, which may promote tumor
growth. Research indicates that T. gondii can increase tumor cell
proliferation by downregulating antitumor genes such as PTEN and FoxO1. This
suggests that T. gondii not only affects immune responses but also
alters critical signaling pathways involved in cell growth and survival.
Future
Research Directions
While
existing studies provide compelling evidence of an association between T.
gondii infection and brain tumors, several critical gaps remain in our
understanding (29, 30). First, it is essential to
establish the causal relationship between infection and tumor development
through well-designed cohort studies that control for confounding factors such
as age, immune status, and other environmental exposures. Additionally, we need
to determine whether the development of tumors creates a favorable environment
for parasite growth or if pre-existing tumors contribute to this process.
Moreover, it is crucial to investigate the biological mechanisms underlying
this association. Future research should focus on elucidating how host immune
responses are altered and how these changes modulate cellular pathways involved
in oncogenesis. Understanding these mechanisms could lead to novel therapeutic
strategies that target T. gondii as a potential risk factor for brain
tumors (31, 32).
Implications
for Public Health
The
implications of these findings extend beyond academic interest and raise
important public health considerations. With T. gondii infections
estimated to affect around one-third of the global population, there is an
urgent need for public health initiatives aimed at reducing exposure to this
parasite (33, 34). Improved cooking and sanitation
practices can help lower transmission risks. Additionally, screening programs
targeting high-risk populations can facilitate early detection and intervention
for those with chronic infections. Understanding the link between infectious
agents like T. gondii and cancer can strengthen our cancer prevention
efforts by identifying modifiable risk factors (35, 36). In
conclusion, the evidence connecting Toxoplasma gondii infection to an
increased risk of brain tumors is compelling, but further exploration is
necessary to fully comprehend its implications for cancer development and
public health (37). The relationship among chronic
infection, immune modulation, and tumor growth presents a complex landscape
that requires interdisciplinary research efforts. By clarifying these
connections, we can enhance prevention strategies and potentially develop targeted
therapies that address both the management of infectious diseases and cancer
treatment.
Author
contribution
PR was
involved in the investigation, methodology, and writing the primary draft of
the manuscript, MEB was involved as a supervisor in all sections of the
manuscript including conceptualization, writing, reviewing and also editing.
All the authors studied the final version of the paper and acknowledged it.
Conflict
of interest
There
is no Conflicts of interest/competing interests.
Funding
There
is no funding.
References
1. Zhao X-Y, Ewald SE. The
molecular biology and immune control of chronic Toxoplasma gondii
infection. The Journal of clinical investigation. 2020;130(7):3370-80.
2. Del Pino LEB, Zanón-Moreno V. Systematic
Review on the Relationship between Toxoplasmosis and Mental Disorders. Actas
Españolas de Psiquiatría. 2024;52(2):149.
3. de Haan L, et al. Association of Toxoplasma
gondii seropositivity with cognitive function in healthy people: A
systematic review and meta-analysis. JAMA psychiatry. 2021;78(10):1103-12.
4. Oncu-Oner T, Can S. Meta-analysis of the
relationship between Toxoplasma gondii and schizophrenia. Annals of
parasitology. 2022;68(1).
5. Cossu G, et al. Association between
toxoplasmosis and bipolar disorder: A systematic review and meta-analysis.
Journal of Psychiatric Research. 2022;153:284-91.
6. Ortiz-Guerrero G, et al. Pathophysiological
mechanisms of cognitive impairment and neurodegeneration by Toxoplasma
gondii infection. Brain sciences. 2020;10(6):369.
7. Pittman KJ, Knoll LJ. Long-term
relationships: the complicated interplay between the host and the developmental
stages of Toxoplasma gondii during acute and chronic infections.
Microbiology and molecular biology reviews. 2015;79(4):387-401.
8. Wang M, Jiang W. Virulence evolution of Toxoplasma
gondii within a multi‐host system. Evolutionary Applications.
2023;16(3):721-37.
9. Ihara F, et al. Changes in neurotransmitter
levels and expression of immediate early genes in brain of mice infected with
Neospora caninum. Sci Rep. 2016;6(1):23052.
10. Yang L, et al. Toxoplasma gondii
infection positively associated with schizophrenia: Evidences from UK Biobank
cohort and case-controlled studies. Journal of Psychiatric Research.
2024;175:243-50.
11. Maisarah A, et al. Association between
infection with Toxoplasma gondii and psychiatric disorders. Folia
Parasitologica. 2022;69:1-10.
12. Ademe M, et al. Is latent Toxoplasma
gondii infection associated with the occurrence of schizophrenia? A
case-control study. PLoS One. 2022;17(6):e0270377.
13. Li Y, et al. Chronic Toxoplasma gondii
infection induces anti-N-methyl-d-aspartate receptor autoantibodies and
associated behavioral changes and neuropathology. Infection and immunity.
2018;86(10):10.1128/iai. 00398-18.
14. Steffen J, et al. Type 1 innate lymphoid
cells regulate the onset of Toxoplasma gondii-induced neuroinflammation.
Cell Reports. 2022;38(13).
15. Laing C, et al. Noradrenergic signaling and
neuroinflammation crosstalk regulate Toxoplasma gondii-induced
behavioral changes. Trends in Immunology. 2020;41(12):1072-82.
16. Tu X-k, et al. GLP-1R agonist liraglutide
attenuates inflammatory reaction and neuronal apoptosis and reduces early brain
injury after subarachnoid hemorrhage in rats. Inflammation. 2021;44:397-406.
17. Chen L, et al. Adenosine, bridging chronic
inflammation and tumor growth. Frontiers in Immunology. 2023;14:1258637.
18. Thirugnanam S, et al. Possible role of Toxoplasma
gondii in brain cancer through modulation of host microRNAs. Infectious
agents and cancer. 2013;8:1-6.
19. Jung YY, et al. Pyrimethamine modulates
interplay between apoptosis and autophagy in chronic myelogenous leukemia
cells. International Journal of Molecular Sciences. 2021;22(15):8147.
20. Matta SK, et al. Toxoplasma gondii
infection and its implications within the central nervous system. Nature
Reviews Microbiology. 2021;19(7):467-80.
21. Ammar AM, et al. Correlation between
toxoplasmosis and schizophrenia in Egyptian patients and its impact on dopamine
serum levels. Acta Tropica. 2024;256:107263.
22. Omidian M, et al. Acute toxoplasmosis can
increase serum dopamine level. Journal of Parasitic Diseases. 2022:1-6.
23. Li Y, et al. Persistent Toxoplasma Infection
of the Brain Induced Neurodegeneration Associated with Activation of Complement
and Microglia. Infect Immun. 2019;87(8).
24. Abdulai-Saiku S, et al. Behavioral
manipulation by Toxoplasma gondii: Does brain residence matter? Trends
in parasitology. 2021;37(5):381-90.
25. Sugden K, et al. Is Toxoplasma gondii
infection related to brain and behavior impairments in humans? Evidence from a
population-representative birth cohort. PLoS One. 2016;11(2):e0148435.
26. Webster JP, et al. Toxoplasma gondii
infection, from predation to schizophrenia: can animal behaviour help us
understand human behaviour? J Exp Biol. 2013;216(1):99-112.
27. Mirzaeipour M, et al. Evaluation of the
tyrosine and dopamine serum levels in experimental infected BALB/c mice with
chronic toxoplasmosis. Journal of Parasitology Research. 2021;2021(1):5511516.
28. Virus MA, et al. Neurological and
neurobehavioral disorders associated with Toxoplasma gondii infection in
humans. Journal of parasitology research. 2021;2021(1):6634807.
29. Erickson LD, et al. Association between Toxoplasma
gondii seropositivity and serointensity and brain volume in adults: A
cross-sectional study. PLoS One. 2021;16(2):e0245994.
30. Jung B-K, et al. Exosomal miRNA-21 from Toxoplasma
gondii-infected microglial cells induces the growth of U87 glioma cells by
inhibiting tumor suppressor genes. Sci Rep. 2022;12(1):16450.
31. Asgari Q, et al. Toxoplasma gondii
infection in patients with brain tumors in Southern Iran: a case-control study.
Journal of Parasitic Diseases. 2023;47(2):291-6.
32. Alim M, et al. Seroprevalence of Toxoplasma
gondii in patients receiving cancer treatment. Cumhuriyet Medical Journal.
2018;40(1):19-24.
33. Lima TS, Lodoen MB. Mechanisms of human
innate immune evasion by Toxoplasma gondii. Frontiers in cellular and
infection microbiology. 2019;9:103.
34. Teimouri A, et al. Role of Toxoplasma
gondii IgG avidity testing in discriminating between acute and chronic
toxoplasmosis in pregnancy. Journal of clinical microbiology.
2020;58(9):10.1128/jcm. 00505-20.
35. Egan KM, et al. Prospective investigation of
polyomavirus infection and the risk of adult glioma. Sci Rep. 2021;11(1):9642.
36. Mao F, et al. Seroprevalence and risk factors
of Toxoplasma gondii infection among high-risk populations in Jiangsu
Province, Eastern China. Frontiers in Cellular and Infection Microbiology.
2021;11:783654.
37. Rostami A, et al. Does latent Toxoplasma
infection have a protective effect against developing multiple sclerosis?
Evidence from an updated meta-analysis. Transactions of The Royal Society of
Tropical Medicine and Hygiene. 2022;116(11):996-1006.