
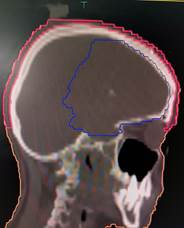
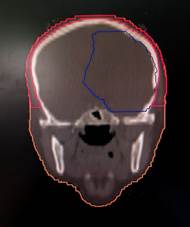
Figure 1. shows scalp contours in axial, coronal,
and sagittal planes.
Scalp dosimetry as a predictor of radiation-induced
alopecia in primary brain tumours: a retrospective study from a tertiary cancer
centre in South India
Lalitha Nageshwari S 1,
Govardhan HB 1 *, Ibrahim
Khaleel 1, Vijayath BR 1, Akshay KT 1,
Priyadarshini T 1, Sahana R 1
1 Department of Radiation Oncology, Kidwai Memorial Institute of
Oncology, Bengaluru- 560029, Karnataka, India
* Corresponding Author:
Govardhan HB
* Email: govardhanhb@gmail.com
Abstract
Introduction: Radiotherapy is
essential in treating primary brain tumours, but radiation-induced alopecia
(RIA) remains a common side effect that significantly affects patients' quality
of life (QOL). With its psychosocial impact on self-image, emotional
well-being, and social interactions, alopecia warrants focused attention. This
study aims to evaluate the scalp as an organ at risk by defining dose
constraints that minimize RIA while maintaining optimal target coverage.
Materials and methods: A retrospective analysis was conducted on 70 patients with primary
brain tumours who received focal cranial radiotherapy between January 2022 and
December 2024. Scalp dose-volume histograms (DVHs) were generated from
treatment planning systems, and the mean scalp dose (D mean), maximum scalp
dose (D max), median volume of scalp, volume of scalp receiving ≥ 30 Gy
(V30Gy), dose received by 20cc (D20cc), and 30cc (D30cc) scalp volume were
recorded. RIA was graded according to the Common Terminology Criteria for
Adverse Events (CTCAE) version 5.0. ROC statistical analysis was performed to
evaluate the predictive value of scalp dosimetric parameters for RIA severity.
Results: The median age of the cohort was 57 years, with a male-to-female
ratio of 1.08:1. The median D max, D mean, V 30 Gy, D20cc were 60.4 Gy,
17.5 Gy, 19.2%, and 46.4 Gy, respectively. Grade 2 and higher RIA was observed
in 63% of patients. V30Gy, either independently or in combination with Scalp D
mean, was identified as a significant predictor of Grade 2 or higher RIA.
Conclusion: Optimising scalp dose parametric during radiotherapy planning may
help mitigate RIA and improve QOL.
Keywords: Scalp dosimetry, Radiotherapy-induced alopecia, Primary brain tumours,
VMAT, QOL
Introduction
Hair is an integral part of physical appearance and self-image, often
influencing self-esteem. As a result, radiation-induced alopecia (RIA) can lead
to significant psychological distress, including feelings of shame, depression,
and social isolation due to the stigma associated with hair loss. RIA may be temporary; it tends to become
persistent with increasing radiation dose (3) and can continue to progress long
after the cessation of radiotherapy.
Management strategies focus on both prevention and treatment.
Prophylactic measures emphasise patient education on proper scalp hygiene,
while topical corticosteroids are used to manage scalp dermatitis, and topical
antibiotics are prescribed to treat infections. In cases where skin reactions
become severe, brief treatment interruptions may be necessary to allow the skin
to heal.
A promising approach to reduce these side effects involves limiting
radiation exposure to the scalp by delineating it as an organ at risk (OAR) and
incorporating dose constraints during radiotherapy planning. However, reduced
scalp toxicity should not be at the expense of compromised target coverage or
exposure of critical brain structures, including the brainstem, optic nerves,
and optic chiasm beyond their respective tolerances (4)
This study aims to highlight and address a significant lacuna in the
existing literature concerning the prediction of radiation-induced alopecia
based on scalp dosimetry and its potential for prevention. We aim to evaluate
the feasibility and clinical relevance of integrating scalp-sparing techniques
into routine radiotherapy planning without compromising target coverage or
treatment delivery.
Materials and methods
We retrospectively identified patients with
primary brain tumours who underwent surgical resection followed by adjuvant
radiotherapy with curative intent at our institution between 2022 and 2024. The
study included adult patients diagnosed with primary intracranial neoplasms,
specifically gliomas, meningiomas, and medulloblastomas. All patients received
conventional volumetric modulated arc therapy (VMAT) without scalp-sparing
optimization, to a total dose of 54–60 Gy in 30 fractions over 6 weeks (5 days
per week), with concurrent chemotherapy administered when clinically indicated.
Patients with brain metastases and pediatric brain tumours were excluded from
the study.
Contouring of target
volumes, scalp and other OARs
CT simulation scans were obtained from the vertex to the C7 vertebrae,
with a slice thickness of 3 mm. The European Organization for Research and
Treatment (EORTC) guidelines were used to generate the CTV, GTV, and PTV
contours. According to EORTC guidelines, the gross tumour volume (GTV) is
defined as the enhancing lesion observed on T1 post-contrast MRI, along with the
postoperative surgical cavity. The GTV is expanded by 1.5 to 2 cm to create the
clinical target volume (CTV). which is then edited from the natural barriers
hindering tumour growth, such as the bones, tentorium, and falx. The planning
target volume (PTV) is then generated by geometrically expanding the CTV by 3
mm. PTV was prescribed doses ranging from 54 Gy to 60 Gy in 30 fractions at
1.8-2 Gy per fraction (5). The scalp, a layered structure directly beneath the
cranial skin surface, was delineated. This scalp contour was extended caudally
to the level of the foramen magnum (6) as depicted in Figure 1 given below.



Figure 1. shows scalp contours in axial, coronal,
and sagittal planes.
Intracranial OARs, namely the brainstem, optic chiasm, optic tract,
optic nerves, lens, and eye, were also delineated.
Radiation planning and
dosimetry
Patients were simulated in a supine position, with thermoplastic masks
used to immobilise the head and neck areas in a neck-neutral position. The
Volumetric Modulated Arc Therapy (VMAT) technique was utilised for treatment
planning. All plans were generated using either the Eclipse version 13.7
(VARIAN) or the Monaco version 6.1.4 (ELEKTA) treatment planning systems with 6
MV photons. Each plan incorporated 1–2 non-coplanar arcs. Plans were optimised
to ensure that 95% of the PTV received 100% of the prescribed dose.
The prescribed dose constraints for organs at risk were:
Optic nerves D max < 54 Gy, Optic Chiasm D max < 54 Gy, Brainstem
D max < 54 Gy, Lens D max < 10 Gy, and Cochlea D max < 45 Gy (7). Scalp-specific dose parameters followed were:
Scalp D mean < 20 Gy, Scalp D20cc < 50 Gy, Scalp D30cc < 40 Gy (8).
Adequate PTV coverage was prioritised over scalp dose constraints.
The pattern of appearance of RIA was clinically observed at weekly
intervals during radiotherapy in review outpatient clinics; while grading and
documentation were done at the 3rd and 6th month follow-up visits
post-radiotherapy, using the Common Terminology Criteria for Adverse Events
(CTCAE) version 5.0. Alopecia severity was graded as follows:
●
Grade 1: Hair loss of <50% of normal for that individual that is not
obvious from a distance but only on close inspection
●
Grade 2: Hair loss of ≥50% normal for that individual that is
readily apparent to others
●
Grade 3: Complete hair loss
Results
Patients receiving focal brain radiotherapy at the Department of
Radiation Oncology at Kidwai Memorial Institute of Oncology between 2022 and
2024 were selected for our study.
The median age of the cohort was 57 years (range: 32 to 77 years), with
52 per cent male and 48 per cent female patients. Among the analysed patient
cohort, 84 percent had a diagnosis of glioma, of which 13 per cent had
low-grade glioma, while 71 per cent had high-grade glioma. Additionally, 10 per
cent of the cohort had medulloblastoma, whereas the remaining 6 per cent had
meningioma. Concerning radiotherapy, 70 percent received 60 Gy in 30 fractions,
while 30 percent received 54 Gy in 30 fractions. Table 1 given below summarises
these baseline patient characteristics.
Table 1. shows the baseline patient characteristics.
|
Baseline characteristics |
Median Value |
|
Age |
57 years |
|
Gender |
Percentage |
|
Male |
52% |
|
Female |
48% |
|
Radiotherapy Dose |
Percentage |
|
54 Gy |
30% |
|
60 Gy |
70% |
|
Diagnosis |
Percentage |
|
Glioma |
84% |
|
Low grade |
13% |
|
High grade |
71% |
|
Medulloblastoma |
10% |
|
Meningioma |
6% |
These patients were routinely followed up in the clinic weekly during
radiotherapy and subsequently at regular intervals, where RIA was assessed.
Grade 2 and higher RIA was observed in 63% of patients, while Grade 1 RIA was
observed in 37% of patients, respectively, as per CTCAE v5.0 grading which is
represented in Figure 2.

Figure 2. visually depicts the RIA severity, showing
Grade 1 and Grade 2 and higher, respectively, in representative patients.
These values reflect aggregate assessments recorded at the third- and
sixth-month follow-ups; however, the subjective perception of alopecia was
reported by patients at a median of two weeks after the initiation of
radiotherapy. Retrospectively, patients were analysed for scalp dosimetric
parameters. Table 2 given below summarises the various scalp dosimetric
parameters.
Table 2. shows Scalp dosimetric parameters.
|
|
Median Value |
|
|
Volume |
382.7cc |
|
|
Dmax |
60.4 Gy |
|
|
Dmean |
17.5 Gy |
|
|
|
Median Value |
|
|
|
cc |
% |
|
V30cc |
70.1 |
19.2 |
|
V40cc |
35.2 |
8.5 |
|
|
Median Value |
|
|
D20cc |
46.4 Gy |
|
|
D30cc |
42.2 Gy |
|
The median scalp volume measured was 382.7 cc (ranging from 166.518 to
573.498 cc). The median scalp D max was 60.4 Gy, while the median scalp D mean
was 17.5 Gy. Median scalp D20cc and D30cc were 46.4 Gy and 42.2 Gy,
respectively. Additionally, the median volumes of the scalp receiving 30 Gy and
40 Gy were 70.1 cc (19.2%) and 35.2 cc (8.5%), respectively. The various
planning parameters have been depicted in Figures 3 and 4 as follows.
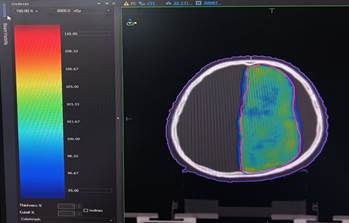 Figure 3. is a visual depiction of scalp dose in a
representative patient via a VMAT plan. The scalp is represented by the purple
colour contour, and PTV is demarcated by the red contour. Dose wash encompasses
an area covered by the 95% iso-dose line.
Figure 3. is a visual depiction of scalp dose in a
representative patient via a VMAT plan. The scalp is represented by the purple
colour contour, and PTV is demarcated by the red contour. Dose wash encompasses
an area covered by the 95% iso-dose line.
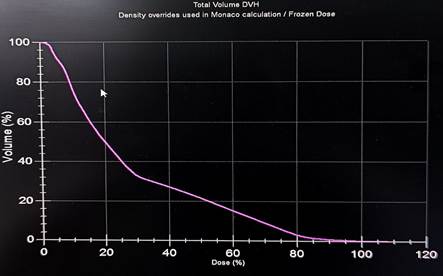 Figure 4. shows a scalp dose volume
histogram (DVH).
Figure 4. shows a scalp dose volume
histogram (DVH).
Descriptive statistics were used to summarise patient characteristics.
The predictive value of scalp dosimetric parameters for RIA severity was
assessed using Receiver Operating Characteristic (ROC) analysis, specifically
the Area Under the Curve (AUC). ROC curve analysis was conducted to assess the
predictive performance of various scalp dosimetric parameters (e.g., Dmean,
Dmax, V10, V20) with alopecia outcomes. The analysis was performed using IBM
SPSS Statistics version 30.0.0, which offers advanced ROC analysis tools. The
AUC was calculated along with 95% confidence intervals (CIs) to quantify
the discriminative ability of each parameter. Optimal threshold values were
determined using the Youden index (J = Sensitivity + Specificity − 1),
which identifies the point that maximizes the balance between sensitivity and
specificity. A p-value < 0.05 was considered statistically significant.
Direct statistical comparison between individual ROC curves (e.g., Dmean
vs. V20) was not performed, as each dosimetric parameter represents a distinct
physical quantity with different biological implications. Therefore, the ROC
curves were interpreted independently to respect the variable-specific nature
of the dosimetric data.
It was observed that V30Gy demonstrated the highest predictive value for
Grade 2 or higher radiation-induced alopecia (RIA), with an AUC of 0.604 (95% CI: 0.431–0.777, p = 0.258).
Using a cut-off value of 28.140%,
V30Gy had a sensitivity of 37.0% and a specificity of 93.8%, indicating a
strong ability to correctly identify patients who are unlikely to develop Grade
2 or severe RIA. Similarly, Scalp D mean (Gy) exhibited a moderate predictive
ability, with an AUC of 0.557 (95%
CI: 0.388–0.726, p = 0.530). At a cut-off value of 20.210 Gy, Scalp D mean had a sensitivity of 48.3% and a
specificity of 81.3%, suggesting a more balanced ability to identify patients
at risk of developing Grade 2 or higher RIA. Although neither parameter reached
statistical significance (p > 0.05), their AUC values and high specificity
indicate that scalp dosimetry could play a potential role in predicting Grade 2
or higher RIA severity
Discussion
Although the mechanisms behind radiotherapy-induced alopecia (RIA) are
not fully understood, significant damage from radiotherapy can affect both the
epithelial stem cells in the bulge region and the rapidly dividing matrix cells
in the hair follicle bulb. This damage to these critical cells preferentially
induces anagen effluvium, a type of hair loss where hair in the growth phase
(anagen) is shed prematurely. This hair loss is consistent with nonspecific
scarring alopecia (9). Typically, this process begins within 2–3 weeks after
the initiation of radiotherapy. Hair follicle radiosensitivity is also
dependent on the hair cycle stage: anagen matrix cells are more radiosensitive
than telogen matrix cells due to relative differences in proliferation rates. A
dose of 3 Gy produces complete, reversible anagen alopecia, whereas permanent
alopecia begins to occur at 5 Gy (10). Complete hair regrowth generally occurs
2−4 months after irradiation in the reversible type of radiation-induced
alopecia (11). In certain patients, RIA
may continue to progress well beyond the completion of radiotherapy (12). This
observation suggests the initiation of a chronic pathogenic cascade that
extends far beyond the early phase of radiation-induced skin and hair follicle
damage. Also considering that anagen hair follicles lie about 4–5 mm deep
embedded within human scalp skin, dose fraction sizes and total cumulative
doses have a direct effect on RIA (13). Due to the lack of reliable data on
scalp dosimetry, clinicians often have difficulty accurately predicting and
explaining the likelihood of alopecia to patients, even after carefully
reviewing treatment plan metrics.
The results of our study suggest that while these dosimetric factors may
have individual relevance, they may not independently serve as strong
predictors of severe RIA. However, combining multiple parameters could enhance
predictive accuracy, supporting the integration of scalp dose optimisation into
radiotherapy planning to reduce the risk of radiation-induced alopecia.
This study has several limitations that warrant consideration. First,
the retrospective design inherently introduces the potential for selection bias
and limits control over confounding variables such as baseline scalp condition,
prior treatments, and comorbidities that may influence the risk of alopecia.
Second, the sample size was relatively small, which may reduce the statistical
power of the findings and limit their generalizability. While the study
identified dosimetric thresholds predictive of alopecia, these results should
be interpreted with caution and validated in larger, prospective cohorts.
Additionally, the assessment of alopecia was based on available clinical
documentation and grading scales, which may be subject to interobserver
variability. Finally, although ROC curve analysis provided insight into the
predictive performance of individual dosimetric parameters, multivariate
analysis was not performed to adjust for potential confounding factors such as
age, chemotherapy exposure, or concurrent treatments, all of which may
independently contribute to hair loss.
Although our study is retrospective, scalp dosimetric parameters did not
compromise planning target volume (PTV) coverage or pose a risk to adjacent
critical organs. This highlights the potential for Scalp Sparing
Volumetric-Modulated Arc Therapy (SSV) as a feasible strategy in radiotherapy.
Further studies with larger sample sizes and multivariate analysis may refine
predictive models, establish clinically actionable scalp dose constraints, and
support routine implementation in clinical practice.
Conclusion
Author
contribution
GHB and IKh conceptualization and
validation, LNS and GHB methodology and Software, LNS
formal analysis and writing original draft, LNS, VBR, AKT,
PT and SR investigation, VBR, AKT, PT and SR
resources acquisition and data curation, LNS, GHB and IKh
reviewing and editing and project administration, LNS and VBR
visualisation, GHB supervision.
Conflict of
interest
The author
declares no conflict of interest.
Funding
There is no
funding.
References
1. Freites-Martinez A,
Shapiro J, van den Hurk C, Goldfarb S, Jimenez JJ, Rossi AM, et al. Hair
disorders in cancer survivors. J Am Acad Dermatol. 2019;80(5):1199–1213.
2. Phillips GS, Freret ME,
Friedman DN, Trelles S, Kukoyi O, Freites-Martinez A, et al. Assessment and
treatment outcomes of persistent radiation-induced alopecia in patients with
cancer. JAMA Dermatol. 2020;156(9):963–972.
3. Lawenda BD, Gagne HM,
Gierga DP, Niemierko A, Wong WM, Tarbell NJ, et al. Permanent alopecia after
cranial irradiation: dose-response relationship. Int J Radiat Oncol Biol Phys.
2004;60(3):879–887.
4. Briere TM, McAleer MF,
Levy LB, Yang JN. Sparing of normal tissues with volumetric arc radiation
therapy for glioblastoma: single institution clinical experience. J Radiat
Oncol. 2017;12(1):81.
5. Niyazi M, Andratschke
N, Bendszus M, Chalmers AJ, Erridge SC, Galldiks N, et al. ESTRO-EANO guideline
on target delineation and radiotherapy details for glioblastoma. J Radiother
Oncol. 2023;184:109663.
6. Miller R, Song A, Ali
A, Niazi M, Bar-Ad V, Martinez N, et al. Scalp-sparing radiation with
concurrent temozolomide and tumor treating fields (SPARE) for patients with
newly diagnosed glioblastoma. J Front Oncol. 2022;12:896246.
7. Scoccianti S, Detti B,
Gadda D, Greto D, Furfaro I, Meacci F, et al. Organs at risk in the brain and
their dose-constraints in adults and in children: a radiation oncologist’s
guide for delineation in everyday practice. J Radiother Oncol. 2015;114(2):230–238.
8. Song A, Bar-Ad V,
Martinez N, Glass J, Andrews DW, Judy K, et al. Initial experience with scalp
sparing radiation with concurrent temozolomide and tumor treatment fields
(SPARE) for patients with newly diagnosed glioblastoma. J Neurooncol.
2020;147(3):653–661.
9. Malkinson FD, Keane JT.
Radiobiology of the skin: review of some effects on epidermis and hair. J
Invest Dermatol. 1981;77(1):133–138.
10. Katz SI, Barbara A,
Gilchrest AS, Paller DJ. Fitzpatrick’s dermatology in general medicine. In:
Wolff K, Goldsmith LA, editors. New York: McGraw-Hill; 2008.
11. Wen CS, Lin SM, Chen Y,
Chen JC, Wang YH, Tseng SH. Radiation-induced temporary alopecia after
embolization of cerebral arteriovenous malformations. J Clin Neurol Neurosurg.
2003;105(3):215–217.
12. Hymes SR, Strom EA, Fife
C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment.
J Am Acad Dermatol. 2006;54:28–46.
13. De Puysseleyr A, Van De
Velde J, Speleers B, Vercauteren T, Goedgebeur A, Van Hoof T, et al.
Hair-sparing whole brain radiotherapy with volumetric arc therapy in patients
treated for brain metastases: dosimetric and clinical results of a phase II
trial. J Radiat Oncol. 2014 Dec;9:1–8.