An examination of
the cytomorphological trends in tuberculous lymphadenitis at a tertiary care
hospital of southern Assam
Sukanya
Choudhury 1, Payel Hazari 1*, Nicky Choudhury 1,
Shah Alam Sheikh 1
1 Department of Pathology, Silchar Medical
College and Hospital, India
Corresponding
Authors: Payel Hazari
* Email: hazari.payel2@gmail.com
Abstract
Introduction: Tuberculous lymphadenitis is a leading cause of
lymph node enlargement accounting for 195 per 1,00,000 population in India.
Fine needle aspiration cytology (FNAC) is rapid and economical compared to
other tests and thus plays a crucial role in diagnosing this condition. It
prevents unnecessary biopsy of lymph nodes and it can be used for collection of
material for cytomorphological and bacteriological examination. This study
aimed to assess the cytomorphological patterns of tuberculous lymphadenitis and
correlate them with Ziehl-Neelsen (ZN) staining. Primary
aim is to assess the cytomorphological patterns of tubercular lymphadenitis and
to correlate with the bacteriological results using ZN staining.
Materials and methods: The FNAC results of 100 cases diagnosed with tuberculous lymphadenitis
over a period of one year were analyzed from the
cytopathology section of Silchar Medical College and
Hospital. The findings were classified into three patterns: pattern A -
epithelioid granuloma in absence of caseous necrosis, pattern B - epithelioid
granuloma with caseous necrosis, and pattern C - caseous necrosis in absence of
epithelioid granuloma. The cytomorphological patterns were then correlated with
acid-fast bacilli (AFB) positivity.
Results: In individuals between the ages of 21 and 30,
tuberculous lymphadenitis was predominantly observed. The cervical lymph node
(92%) was the most frequently affected area. Among the different patterns of
the condition, Pattern B, which is characterized by the presence of epithelioid
granuloma along with caseous necrosis, was found to be the most common (53%).
In contrast, Pattern C, which is marked by caseous necrosis without the
presence of epithelioid granuloma, exhibited the highest positivity for acid-fast
bacilli (80%). The difference in AFB positivity among the patterns was
statistically significant (P-value= 0.0003).
Conclusion: FNAC is an effective and economical
diagnostic tool for tuberculous lymphadenitis, particularly in resource-limited
settings. The study found that Pattern B (epithelioid granuloma with caseous
necrosis) was the most common, while Pattern C (caseous necrosis without
epithelioid granuloma) exhibited the highest AFB positivity. FNAC, combined
with ZN staining, enhances the accuracy of tuberculosis diagnosis, minimizing
the need for invasive biopsies. Given the high prevalence of tuberculosis, FNAC
should be the first-line investigation for patients presenting with superficial
lymphadenopathy, ensuring timely diagnosis and treatment.
Keywords: Cytomorphological patterns, Tuberculous lymphadenitis, Ziehl-Neelsen (ZN) staining, Acid-fast bacilli
Introduction
Tuberculosis is very common in India accounting for
195 per 1,00,000 population (1). Lymphadenitis is the most common clinical
presentation of extrapulomonary tuberculosis with
tubercular lymphadenitis accounting for 43% cases in developing countries like
India. Diagnosis of tuberculosis can be done by various diagnostic methods as
fine needle aspiration cytology, biopsy, Acid fast bacilli culture and polymerase
chain reaction .(1) FNAC is a widely used cytological
technique for diagnosing tubercular lymphadenitis offering a sensitivity and
specificity of 88-96% and is most cost effective and rapid compared to other
tests like AFB culture and PCR (2). FNAC can detect tubercular lymphadenitis
even in cases where the bacterial load is low or when the disease is in its
early stages.
The sensitivity increases significantly when FNAC is
combined with Ziehl-Neelsen staining for acid-fast bacilli .The presence of epithelioid cell granulomas,
caseous necrosis, and Langhans giant cells on cytology strongly points to a
tubercular etiology, leading to accurate diagnosis
and exclusion of other causes of lymphadenopathy (e.g., lymphoma, metastatic
carcinoma).
It also minimises unnecessary lymph node biopsies and
providing material for cytopathological and bacteriological analysis (3).
Its ability to provide rapid cytological and
bacteriological insights makes it invaluable in early diagnosis and management
of TB, especially in high-burden and resource-limited settings. Combining FNAC
with other tests like ZN staining further enhances its diagnostic yield and
clinical utility.
To diagnose tuberculosis cytologically, the
identification of epithelioid cell granuloma with or without necrosis is
essential (4-7). A confirmed diagnosis is achieved by identifying the presence
of acid-fast bacilli by doing ZN staining.
This study undertakes a comprehensive examination of
the cytomorphological patterns of tubercular lymphadenitis, with a specific
focus on correlating FNAC findings with bacteriological confirmation via ZN
staining. This study aims to thoroughly analyze the
cytomorphological patterns
observed in tubercular lymphadenitis using FNAC. The goal is to
identify and describe the specific cellular changes and features such as
granulomas, caseous necrosis, and giant cells seen in FNAC smears from patients
with tubercular lymphadenitis.
Additionally, the study seeks to establish a
correlation between the FNAC findings and bacteriological confirmation of
tuberculosis through ZN staining. A positive correlation would mean that the
cytological features identified in FNAC are consistent with the presence of AFB
as confirmed by ZN staining, thereby strengthening the diagnostic value of FNAC
for tubercular lymphadenitis.
Materials
and methods
The present study was undertaken to assess the
cytomorphological patterns of tubercular lymphadenitis and to correlate with
the bacteriological results using Ziehl-Neelsen (ZN)
staining.
This study was conducted in the Department of
Pathology, Silchar Medical College and Hospital, Silchar, Assam, India. The Institutional Ethics Committee
approved the study (No.SMCH/ETHICS/M2/2024/46)
on 30/08 /2024. The study is compliant with the Helsinki Declaration’s ethical
guidelines.
Study period
1 year period: From August 2023 to July 2024
Source of data and sample size
100 patients were diagnosed with tuberculous
lymphadenitis based on FNAC of peripheral lymphadenopathy and the data were
collected from the registers of the cytopathology section of the Pathology
department of Silchar Medical College and Hospital.
The FNAC slides stained with MGG and ZN stains were reviewed.
Inclusion criteria
Clinically suspected
cases of tubercular lymphadenitis and those not on anti
tuberculosis treatment (ATT)
Exclusion
criteria
·
On
anti-tubercular treatment.
·
FNAC samples yielding inadequate or
non-diagnostic material.
Parameters
studied
·
Detailed clinical history and other investigations of the patients were
recorded after taking patient consent.
·
Hospital records of the patients.
·
Microscopic examination of the FNAC slides.
This study, conducted from 2023 to 2024 at Silchar Medical College and Hospital, Assam, involved 100
patients diagnosed with tuberculous lymphadenitis through FNAC of peripheral
lymphadenopathy. Data were collected from cytopathology registers, and FNAC
slides stained with MGG and ZN stains were reviewed. Characteristic
cytomorphological features, including epithelioid granuloma with or without
caseous necrosis and ZN staining, confirmed the diagnosis.
The following techniques were performed in the
preparation of FNAC (Fine Needle Aspiration Cytology) slides:
Fine needle aspiration cytology (FNAC)
procedure
1. Preparation for the procedure
1.1 Materials required
·
Syringe: Disposable plastic syringe (10-20 ml
capacity)
·
Needle: 20-22 gauge
needle (length varies based on tumor site)
·
Antiseptic solution: For cleaning the puncture site
·
Glass slides: For smear preparation
·
Staining reagents: May-Grunwald Giemsa (MGG) and Ziehl-Neelsen (ZN) stains
1.2 Patient Preparation
·
The patient is positioned comfortably and reassured to reduce
anxiety.
·
No local anesthesia is required as FNAC is a
minimally invasive procedure.
·
The target mass is localized by palpation, and a suitable puncture site
is chosen.
2. Aspiration procedure
2.1 Needle insertion and aspiration
1. The skin around the target site is
thoroughly cleaned with an antiseptic solution.
2. The lesion is held firmly in place with one
hand while the needle is inserted at the predetermined site.
3. Once the needle enters the lesion, the
syringe plunger is retracted to create negative pressure for aspiration.
4. The needle is moved within the mass (3-4
oscillations in different directions) to obtain an adequate sample.
2.2 Withdrawal and sample handling
5. Before withdrawing the needle, the plunger
is released to equalize pressure, preventing blood contamination.
6. The needle is then removed, and detached from
the syringe, and air is drawn into the syringe to facilitate sample expulsion.
7. The needle is reattached, and the aspirated
material is expelled onto a clean glass slide.
3. Preparation of Smears
3.1 Spreading the aspirate
·
The aspirate is examined macroscopically.
·
Semi-solid aspirates are spread using a thick Burker-type cover slip.
·
Tissue fragments are gently crushed for uniform distribution.
4. Staining Procedure
4.1 May-Grunwald Giemsa (MGG) Staining
1. The air-dried smear is fixed in
methanol.
2. May-Grunwald stain is applied for 5-7
minutes, followed by Giemsa stain for 10-15 minutes.
3. The slide is rinsed with buffered water
until the excess stain is removed.
4. The slide is then air-dried and ready for
microscopic examination.
4.2 Ziehl-Neelsen
(ZN) Staining for Acid-Fast Bacilli (AFB)
4.2.1 Primary staining
1. The air-dried and heat-fixed smear is
flooded with carbol fuchsin stain.
2. The slide is gently heated until steam
appears (without boiling) to ensure dye penetration.
3. It is left to stand for 5-10 minutes to
allow effective staining.
4.2.2 Decolorization
1. The slide is washed with water.
2. It is decolorized using acid-alcohol (20%
sulfuric acid or 3% hydrochloric acid in ethanol) until the red stain fades.
3. The slide is rinsed again with water.
4.2.3 Counterstaining
1. Methylene blue or malachite green is
applied for 1-2 minutes to provide a contrasting background.
2. The slide is washed, air-dried, and ready
for microscopic evaluation.
5. Slide Interpretation and reporting
5.1 Cytomorphological classification
The FNAC results are classified into three patterns:
·
Pattern A: Epithelioid granuloma without necrosis
·
Pattern B: Epithelioid granuloma with necrosis
·
Pattern C: Necrosis without epithelioid granuloma,
with neutrophilic infiltration
5.2 Statistical Analysis
·
Software Used: SPSS (Version 21)
·
Test performed: Chi-square test to correlate
cytomorphological patterns with AFB positivity.
·
Significance level: A p-value < 0.05 was considered
statistically significant.
Results
Out
of the 100 patients studied, 42 (42%) were male and 58 (58%) were female,
indicating a slight female predominance, with a male-to-female ratio of 1:1.4. The
ages of the patients ranged from 1 to 71 years, with an average age of 29.28
years. The disease was most prevalent in the age group of 21 to 30 years,
followed by the 11 to 20 years age group (Table 1). Cervical lymph nodes were
the most commonly affected, involved in 92% of cases, while inguinal lymph
nodes were affected in 8% of cases.
Table
1. The patient
information.
|
|
Total (%)
|
|
Gender
|
Male
|
42
|
|
Female
|
58
|
|
Age group
|
01-10 years
|
8
|
|
11-20 years
|
16
|
|
21-30 years
|
38
|
|
31-40 years
|
13
|
|
41-50 years
|
12
|
|
51-60 years
|
5
|
|
61-70 years
|
6
|
|
71-80 years
|
2
|
Three distinct cytopathological patterns were
identified: Pattern A, which consisted of epithelioid granuloma in the absence
of caseous necrosis; Pattern B, which included epithelioid granuloma with
necrosis; and Pattern C, characterized by caseous necrosis without epithelioid
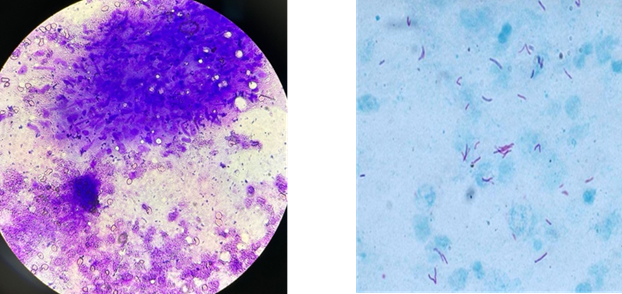
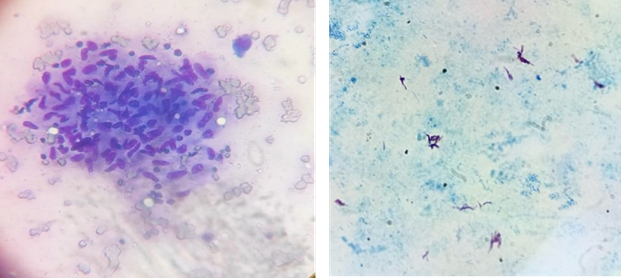
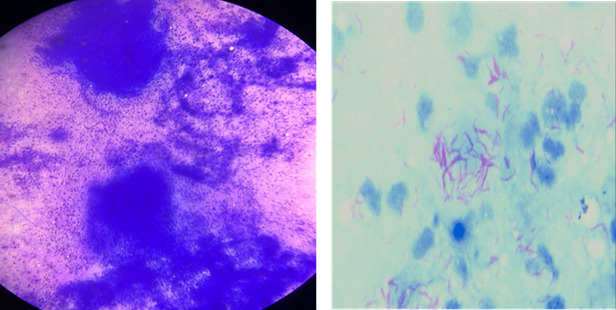
granuloma (refer to Table 2 and Figures 1 A, B, and C).
Among these patterns, Pattern B was the most
prevalent, observed in 53 cases out of 100, representing a partially effective
immune response where granulomas are present, but necrosis indicates tissue
destruction and active bacterial replication.
Pattern A was
the second most prevalent, observed in 42 cases, thus representing a contained
immune response where macrophages are effectively forming granulomas to control
bacterial proliferation.
The least prevalent was pattern C, observed in 5
cases, reflecting a defective immune response where the absence of granuloma
formation allows bacterial proliferation and leads to extensive tissue damage.
A Ziehl-Neelsen stain for acid-fast
bacilli, indicative of tuberculosis, revealed AFB positivity in 47 cases. The
distribution of AFB positivity across the three cytopathological patterns was
as follows: Pattern B had 33 positive cases out of 53 (62.3%), Pattern A had 10
positive cases out of 42 (23.8%), and Pattern C had 4 positive cases out of 5
(80%). Thus the highest percentage of AFB positivity
was seen in Pattern C, where there was no epitheloid
granuloma formation indicating a
ineffective immune response and thus increased bacillary load.
The differences in AFB positivity among the patterns
were statistically significant, with a P value of 0.0003 and a Chi-square value
of 16.211.
The statistically significant differences confirm that
Pattern C is strongly associated with high bacillary load and poor immune
control. The lower AFB positivity in Pattern A reflects an effective immune
response, while the higher AFB positivity in Pattern B and Pattern C reflects
progressive or poorly contained infection.
Pattern B’s predominance suggests that most cases of
tubercular lymphadenitis involve an active immune response with ongoing tissue
destruction.
The high AFB
positivity in Pattern C highlights the need for aggressive antitubercular
therapy in cases where granuloma formation is absent, as these cases are likely
to have higher bacterial loads.
The low AFB
positivity in Pattern A underscores the protective role of granuloma formation
in containing TB infection.
This analysis supports the use of FNAC not only for
diagnosis but also for predicting disease severity and guiding treatment
strategies.
Table
2. Cytomorphological
patterns of tubercular lymphadenitis.
|
Cytopathological pattern
|
Total no. of cases
|
AFB positive cases
|
AFB negative cases
|
AFB positivity (%)
|
|
Pattern B
|
53
|
33
|
20
|
62.3%
|
|
Pattern A
|
42
|
10
|
32
|
23.8%
|
|
Pattern C
|
5
|
4
|
1
|
80%
|
|
Total
|
100
|
47
|
53
|
|
|
|
|
P value=
0.0003
|
|
|
Discussion
FNAC is the primary method
for reliably diagnosing tubercular lymphadenitis in patients who present with
superficial lymphadenopathy, as it is simple, safe, and cost-effective (8). In
our study, the mean age of presentation and the age group exhibiting the
highest incidence of tubercular lymphadenitis is consistent with studies
conducted by Bhatta S et al. and Hemalatha A et al (9,10) , where the most
frequent age group effected was 21-30 years .There was a slight female
predominance(Male: Female-1.4:1) , which aligns with results from Purohit MR, et al (4) (Male: Female- 1.5:1) and
Polesky et al. (female to male ratio of 1.9:1) (16).
The most commonly involved
lymph node in our study was the cervical lymph node(92%)
which corresponds with the study by Bezabith M et
al., which reported a 74.2% involvement of lymph nodes, and the research by
Paliwal N et al., which indicated a 90% involvement rate (8,13).
The most commonly observed
pattern was Pattern B(53%), epithelioid granuloma with
caseous necrosis, which was also the most prevalent in studies by Bhatta S et
al ( 9) (53.17%) and Khanna A et al (14) (50.5%). The
least common pattern in our study was caseous necrosis without epithelioid
granuloma (5%), observed.
The overall positivity for
Acid-Fast Bacilli in our study was 47%. An inverse relationship was found
between AFB positivity and the presence of granuloma: the highest AFB
positivity was seen in smears containing only necrosis and neutrophilic
infiltrates, while the lowest was found in smears with only epithelioid
granulomas. The patient’s cell-mediated immunity triggers a granulomatous
response against tubercle bacilli, resulting in lower AFB positivity in smears
showing epithelioid granuloma without necrosis. In contrast, smears containing
only necrotic material demonstrated higher AFB positivity due to a compromised
immune response and the absence of a granulomatous reaction. Paliwal N et al.
and Bezabith M et al. reported overall AFB positivity
rates of 71% and 59.5%, respectively (9,14,15). The relatively low AFB
positivity in our study may be attributed to the predominance of epithelioid
granuloma with or without necrosis. Repeating Fine Needle Aspiration Cytology
may improve AFB positivity rates. The association between AFB positivity and
the three cytomorphological patterns in our study was statistically significant
(p = 0.0003).
The clinical outcomes can
vary based on the patterns and their findings as discussed below:
1. Granuloma with Caseous
Necrosis: This pattern indicates an active immune response to the infection. It
generally has a favorable prognosis, as it responds
well to anti-tuberculosis therapy (ATT). The presence of granulomas and caseous
necrosis suggests that the body is successfully containing the infection,
leading to a complete resolution with treatment. Relapse rates tend to be low
if the treatment is completed properly.
2. Granuloma without Caseous
Necrosis: This may indicate an early or less severe form of the infection,
where the immune system is managing to contain the bacteria before extensive
tissue damage occurs. The prognosis is still good because these cases usually
respond well to ATT, and there is often less tissue damage to repair. However,
close follow-up is essential to ensure that the infection does not progress to
the caseous necrosis stage.
3. Necrosis without
Granuloma: This pattern may suggest a compromised or ineffective immune
response, as the typical granuloma, which encloses the infection, is absent.
This scenario may arise in cases with a high bacillary load or in
immunocompromised patients. The prognosis for these cases is generally poorer
compared to the other patterns, as there is a higher risk of infection spread
and complications. Treatment response can be slower, and these cases require
more intensive monitoring and longer follow-up to ensure complete resolution of
the infection.
The following limitations must be acknowledged in this
study:
- Restricted
Study Population – The study only
included cases from a single institution (Silchar
Medical College and Hospital), limiting its generalizability to a broader
population. Cases from private hospitals and rural healthcare centers were not considered.
- Exclusion
of Certain Diagnostic Methods –
Patients who underwent additional investigations such as CBNAAT could not
be included due to a lack of traceability, which may have impacted the
study’s ability to compare different diagnostic techniques.
These limitations suggest that while the study
provides valuable insights, a more comprehensive, multi-center
approach could enhance its applicability and accuracy.
Conclusion
This study highlights the diagnostic utility of Fine
Needle Aspiration Cytology (FNAC) in identifying cytomorphological patterns of
tuberculous lymphadenitis and correlating them with bacteriological
confirmation via Ziehl-Neelsen (ZN) staining. The key
findings include:
1. Prevalence and Demographics: Tuberculous lymphadenitis predominantly affects
individuals between 21 and 30 years of age, with a slight female predominance.
The cervical lymph nodes are the most commonly involved (92%).
2. Cytomorphological Patterns: The most prevalent pattern was Pattern B
(epithelioid granuloma with caseous necrosis, 53%), indicating an active immune
response with ongoing tissue destruction. Pattern A (epithelioid
granuloma without necrosis, 42%) reflects a well-contained immune response,
whereas Pattern C (caseous necrosis without granuloma, 5%) suggests a
defective immune response and higher bacillary load.
3. AFB Positivity: The highest AFB positivity (80%) was observed in Pattern C,
indicating poor immune containment, followed by Pattern B (62.3%) and Pattern
A (23.8%). The association between AFB positivity and cytomorphological
patterns was statistically significant (p = 0.0003).
4. Clinical Implications:
o
Pattern B cases respond well to anti-tuberculosis
therapy (ATT) with a favorable prognosis.
o
Pattern A cases require close monitoring to prevent
progression to necrosis.
o
Pattern C cases, with high bacterial loads,
necessitate aggressive ATT and close follow-up due to the risk of
complications.
Implications for Diagnosis and Treatment
·
FNAC should be the first-line diagnostic approach for superficial
lymphadenopathy, particularly in resource-limited settings, as it is minimally
invasive, cost-effective, and provides rapid results.
·
ZN staining enhances diagnostic accuracy, helping distinguish between
different immune responses to tuberculosis.
·
Treatment strategies should be tailored based on cytomorphological
patterns to ensure better disease management and patient outcomes.
·
Further research is needed to validate these findings across multiple
healthcare centers and integrate molecular diagnostic
tools like CBNAAT for enhanced sensitivity.
This study reinforces FNAC’s role in diagnosing
tuberculous lymphadenitis and guiding treatment, ultimately contributing to
better tuberculosis control strategies.
Acknowledgments
The
author would like to extend thanks to the technicians of cytopathology section
for their help. This research received no specific grant from any funding
agencies in the public, commercial or non-profit sectors.
Author
contribution
All the authors of this research paper have
directly participated in the planning, execution, or analysis of the study. SCh
and PH conceived the idea, designed the study, SCh and NCh collected the data, SCh and PH performed
the statistical analysis and wrote the paper. ShASh
guided the research project and reviewed the slides and the literature. All
the authors of this paper have read and approved the final version submitted.
Conflict
of interest
The
authors declare that they have no competing interests.
Funding
There
is no funding agency involved in this research.
References
1. Gupta
A, Kunder S, Hazra D, Shenoy VP, Chawla K. Tubercular lymphadenitis in the 21st
century: A 5-Year single-center retrospective study from South India. Int J Mycobacteriol. 2021 Apr-Jun;10(2):162-165.
2.
Lawn SD, Churchyard G. Epidemiology of HIV-associated tuberculosis. Current
Opinion in HIV and AIDS. 2009 Jul 1;4(4):325-33.
3.
Khajuria R, Goswami KC, Singh K, Dubey VK. Pattern of lymphadenopathy on fine
needle aspiration cytology in Jammu. JK Sci. 2006 Jul;8(3):157-9.
4.
Purohit MR, Mustafa T, Mørkve O, Sviland L. Gender
differences in the clinical diagnosis of tuberculous lymphadenitis—a
hospital-based study from Central India. International Journal of Infectious
Diseases. 2009 Sep 1;13(5):600-5.
5.
Giri S, Singh K. Fine needle aspiration cytology for the diagnosis of
tuberculous lymphadenitis. International Journal of Current Research and
Review. 2012 Dec 15;4(24):124.
6.
Butt T, Ahmad RN, Kazmi SY, Afzal RK, Mahmood A. An update on the diagnosis of
tuberculosis. J Coll Physicians Surg Pak. 2003 Dec;13(12):728-34. PMID:
15569566.
7.
Laishram RS, Devi RK, Konjengbam R, Devi RK, Sharma
LD. Aspiration cytology for the diagnosis of tuberculous lymphadenopathies: A
five-year study. J Indian Acad Clin Med. 2010
Jan;11(1):31-5.
8. Bezabih M, Mariam DW, Selassie SG. Fine needle aspiration
cytology of suspected tuberculous lymphadenitis. Cytopathology. 2002
Oct;13(5):284-90.
9.
Bhatta S, Singh S, Chalise SR. Cytopathological patterns of tuberculous
lymphadenitis: An analysis of 126 cases in a tertiary care hospital. Int. J.
Res. Med. Sci. 2018 Jun;6(6):1898-901.
10.
Hemalatha A, Shruti PS, Kumar MU, Bhaskaran A. Cytomorphological patterns of
tubercular lymphadenitis revisited. Annals of medical and health sciences
research. 2014;4(3):393-6.
11.
Paul PC, Goswami BK, Chakrabarti S, Giri A, Pramanik R. Fine needle aspiration
cytology of lymph nodes-An institutional study of 1448 cases over a five year period. Journal of Cytology. 2004 Oct
1;21(4):187-90.
12.
Gopinathan VP. Tuberculosis in the Indian scene. From a clinician's angle. The
Journal of the Association of Physicians of India. 1989 Aug 1;37(8):525-8.
13.
Nidhi P, Sapna T, Shalini M, Kumud G. FNAC in tuberculous lymphadenitis:
Experience from a tertiary level referral centre.
Indian J Tuberc. 2011 Jul 1;58(3):102-7.
14.
Khanna A, Khanna M, Manjari M. Cytomorphological patterns in the diagnosis of
tuberculous lymphadenitis. International Journal of Medical and Dental
Sciences. 2013;2(2):182-8.
15.
Prasoon D. Acid-fast bacilli in fine needle aspiration smears from tuberculous
lymph nodes. Acta cytologica. 2000;44(3):297-300.
16.
Polesky, Andrea MD, PhD; Grove, William MD; Bhatia, Gulshan MRCP(UK).
Peripheral Tuberculous Lymphadenitis: Epidemiology, Diagnosis, Treatment, and
Outcome. Medicine 84(6):p 350-362, November 2005.