Risk of hepatocellular carcinoma in
adults exposed to oral antidiabetic drugs: a systematic review
Shadman Newaz 1*, Monami
Ahmed 2, Snigdho Hritom
Sil 1, Mst Samanta Hoque 3, Mehbub Hossain 1, Tonima Tabassum Dola 1,
Afia Anjum 1, Sohana Nasrin 4, Ayesha Noor 5
1 Tangail Medical College, Tangail, Bangladesh
2 Department of Anatomy, Medical College for Women and Hospital,
Dhaka, Bangladesh
3 Rajshahi Medical
College, Rajshahi, Bangladesh
4 Sir Salimullah Medical College, Dhaka,
Bangladesh
5 Department of Pharmacy, Jahangirnagar University, Dhaka, Bangladesh
* Corresponding
Author: Shadman Newaz
* Email: shadmannewaz11@gmail.com
Abstract
Introduction: An increasing amount
of clinical research is being conducted on the association between antidiabetic
medications and the outcomes of hepatocellular carcinoma. By offering a
thorough synthesis of the available data and pinpointing topics for further
investigation, this systematic review seeks to assess any possible correlations
between the results.
Materials and methods: According to a registered protocol on the Open Science Framework,
we carried out a systematic review of research published between January 2015
and March 2025. We reviewed several databases to identify English-language
research employing a range of study designs, including observational studies,
cohort studies, and clinical trials. After a thorough screening procedure, 18
studies were chosen from 1089 records. Following the parameters of this study,
the main objective was to summarize the evidence without doing a formal quality
assessment.
Results: Our analysis found possible connections between liver cancer
outcomes and several antidiabetic groups, including insulin, metformin,
thiazolidinediones, sodium-glucose cotransporter 2 (SGLT2) inhibitors, GLP-1
receptor agonists, and DPP-4 inhibitors. Metformin, GLP-1 receptor agonists,
and SGLT2 inhibitors were consistently associated with reduced risk of
hepatocellular carcinoma (HCC) and improved survival outcomes. In contrast,
insulin use in cirrhotic patients was linked to increased all-cause mortality
and higher liver-related complications. Thiazolidinediones showed a
time-dependent protective effect, with longer use correlating with lower HCC
risk. The results suggest that some antidiabetic drugs may affect overall
survival, recurrence rates, and mortality specific to liver cancer. We
discovered that rather than being an initiating factor, the majority of
antidiabetic medications have decreased the risk of liver cancer.
Conclusion: This systematic review contributes to a better understanding of the
complex relationship between antidiabetic medications and liver cancer
outcomes. Important conclusions imply that medical professionals ought to think
about the possible effects of particular antidiabetic medications in liver
cancer patients. More extensive randomised controlled
trials with longer follow-up are advised to elucidate these correlations and
guide treatment recommendations.
Keywords: Antidiabetic drugs, Hepatocellular carcinoma, Liver Cancer, Medication
Associations, Diabetes
Graphical abstract
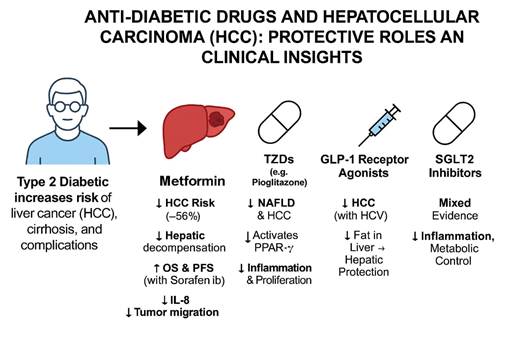
This graphical abstract illustrates the
protective roles of various anti-diabetic medications against hepatocellular
carcinoma (HCC) in patients with type 2 diabetes. The figure begins by
highlighting the increased risk of liver cancer, cirrhosis, and complications
in diabetic individuals. Among the medications, Metformin shows the most
consistent protective effects, including a 56% reduction in HCC risk, improved
survival outcomes, and anti-inflammatory mechanisms. Thiazolidinediones (TZDs)
like pioglitazone reduce NAFLD and HCC through PPAR-γ activation. GLP-1
receptor agonists offer hepatic protection and are particularly beneficial in
HCV-associated liver disease. DPP-4 inhibitors (not shown) also lower HCC risk
in chronic HCV patients. SGLT2 inhibitors exhibit mixed evidence, with
population-dependent benefits through inflammation and metabolic control. This
summary highlights the evolving role of diabetes medications in liver cancer
prevention and management.
Introduction
Type 2 diabetes mellitus (T2DM) is a globally prevalent metabolic
disorder characterized by chronic hyperglycemia resulting from insulin
resistance, impaired insulin secretion, or a combination of both. As of 2021,
more than 537 million people worldwide were estimated to have diabetes, a
number projected to rise significantly over the coming decades (1). The
management of T2DM primarily involves lifestyle modification and
pharmacotherapy with a range of antidiabetic agents that act through diverse
mechanisms—enhancing insulin secretion, improving insulin sensitivity, reducing
hepatic glucose production, or delaying carbohydrate absorption (2).
Liver cancer is a leading cause of cancer-related mortality, with
hepatocellular carcinoma (HCC) being the predominant histological subtype,
accounting for approximately 75–85% of primary liver cancers globally (3). HCC
typically develops in the context of chronic liver diseases, such as hepatitis
B virus (HBV) or hepatitis C virus (HCV) infection, alcoholic liver disease,
and non-alcoholic fatty liver disease (NAFLD), all of which contribute to a
pro-oncogenic hepatic microenvironment characterized by inflammation, fibrosis,
and cellular turnover (4).
Importantly, T2DM has emerged as an independent risk factor for the
development of HCC, with epidemiological studies showing a 2- to 3-fold
increased risk among diabetic individuals compared to non-diabetic
counterparts, even after adjusting for confounding factors such as obesity and
viral hepatitis (5). The biological plausibility of this association is
supported by multiple mechanisms, including chronic hyperinsulinemia, increased
insulin-like growth factor-1 (IGF-1) activity, oxidative stress, lipotoxicity, and chronic low-grade inflammation—all of
which may contribute to hepatic carcinogenesis (6,7).
Antidiabetic medications, while crucial for glycemic control and
prevention of micro- and macrovascular complications, may also influence the
risk of HCC either positively or negatively. For example, metformin, a
biguanide, has demonstrated potential anti-tumorigenic properties through
activation of AMP-activated protein kinase (AMPK), suppression of hepatic
gluconeogenesis, and reduction in insulin levels—mechanisms that may contribute
to decreased HCC risk (8). Conversely, other classes such as insulin, sulfonylureas,
and thiazolidinediones have shown variable or even increased
associations with liver cancer risk, possibly due to their proliferative
effects or impacts on hepatic steatosis and weight gain (9,10).
Adding to the complexity, comorbid conditions frequently seen in
T2DM patients—such as NAFLD, obesity, and chronic viral hepatitis—may interact
with specific medications to modulate the risk of liver carcinogenesis. The
presence of such conditions may alter hepatic drug metabolism, increase
susceptibility to hepatotoxicity, or modify the underlying pathophysiology
leading to cancer development (11,12).
Despite the growing body of literature on the association between
T2DM and liver cancer, previous reviews have often been limited in scope,
focusing either on the general relationship between diabetes and cancer risk or
on isolated drug classes without accounting for confounding comorbidities and
evolving treatment paradigms. Furthermore, with the introduction of newer
classes of antidiabetic drugs—such as GLP-1 receptor agonists and SGLT-2
inhibitors—there is an urgent need to evaluate their long-term hepatic
safety profiles and potential protective effects (13,14).
Objective of the Review
This systematic review aims to provide a comprehensive synthesis of
the available evidence on the relationship between antidiabetic medications and
liver cancer outcomes, particularly hepatocellular carcinoma. Specifically, it
seeks to:
·
Evaluate the impact of individual
antidiabetic drug classes (e.g., metformin, insulin, sulfonylureas, DPP-4 inhibitors,
GLP-1 receptor agonists, SGLT-2 inhibitors) on the incidence, progression,
recurrence, and mortality of liver cancer.
·
Explore the role of underlying
hepatic conditions (e.g., cirrhosis, viral hepatitis, NAFLD) and patient-level
risk factors (e.g., age, obesity, duration of diabetes) in modifying these
associations.
·
Identify potential protective or
harmful effects based on drug type, duration of exposure, and population
subgroups.
·
Provide evidence-based insights to
guide clinical decision-making in the pharmacological management of T2DM in
patients at risk for liver cancer.
By bridging the current knowledge gap and synthesizing diverse data
sources, this review aims to support more informed, personalized, and safer
antidiabetic therapy decisions in patients at risk of hepatocellular carcinoma.
Materials and methods
Study Design and Protocol Registration
This systematic review was conducted in accordance with the
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)
guidelines. The review protocol was prospectively registered on the Open
Science Framework (OSF) to ensure transparency and methodological rigor. The
primary objective was to synthesize existing literature on the relationship
between antidiabetic medications and liver cancer outcomes. Due to anticipated
heterogeneity in study populations, medication types, and outcome definitions,
a narrative synthesis was chosen over a meta-analysis to summarize the
findings.
Eligibility Criteria
Inclusion Criteria:
·
Peer-reviewed articles published
between January 2015 and March 2025.
·
Study designs: Randomized controlled
trials (RCTs), cohort studies, case-control studies, and observational studies.
·
Studies reporting on the impact of
antidiabetic medications (e.g., metformin, insulin, sulfonylureas, SGLT-2
inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, thiazolidinediones) on
liver cancer outcomes.
·
Population: Adults with diabetes
mellitus (type 1 or type 2), with or without pre-existing liver disease.
·
Outcomes: Incidence, progression,
recurrence, survival, or mortality of liver cancer.
·
Published in English.
Exclusion Criteria:
·
Studies published prior to January
2015 due to outdated diagnostic standards and drug classifications.
·
Non-English articles.
·
Conference abstracts, editorials,
commentaries, and study protocols.
·
Studies addressing other cancer
types without specific liver cancer data in relation to antidiabetic use.
·
Animal or in vitro studies.
Search Strategy
A comprehensive electronic search was conducted across the
following databases:
·
PubMed
·
ScienceDirect
·
Cochrane CENTRAL
·
Mendeley
The search strategy combined MeSH terms
and free-text keywords using Boolean operators. Key search terms included:
·
Antidiabetic agents:
"metformin" OR "insulin" OR "sulfonylureas" OR
"DPP-4 inhibitors" OR "GLP-1 receptor agonists" OR
"SGLT-2 inhibitors" OR "thiazolidinediones" OR
"glinides" OR "antidiabetic drugs"
·
Liver cancer: "liver
cancer" OR "hepatocellular carcinoma" OR
"cholangiocarcinoma" OR "liver carcinoma" OR "hepatic
neoplasms"
·
Combined terms:
o
"diabetes
treatment" AND "liver cancer risk"
o
"antidiabetic
side effects" AND "liver cancer survival"
o
"risk
of liver cancer" AND "antidiabetic drugs"
Filters applied included:
·
Publication date from January 2015
to March 2025
·
English language
·
Human subjects only
The initial search was performed on January 26, 2025, with an
update on March 26, 2025. Additionally, the reference lists of relevant reviews
and included studies were manually screened to identify additional eligible
publications.
Study Selection Process
The selection process was conducted using Rayyan, a web-based tool
for systematic review screening. Two reviewers (TD and MSH) independently
screened the titles and abstracts of all retrieved citations. Full texts of
potentially eligible studies were then assessed for inclusion. Any
disagreements were resolved by a third reviewer (SS). A PRISMA flow diagram was
generated to illustrate the screening and selection process.
Data Extraction
A standardized data extraction form was developed in Microsoft
Excel. Extracted variables included:
·
Author(s) and year of publication
·
Country and setting
·
Study design
·
Sample size and characteristics
·
Type(s) of antidiabetic
medication(s) assessed
·
Liver cancer outcomes (incidence,
progression, survival, etc.)
·
Key findings and effect estimates
·
Confounding factors and statistical
methods
Primary extraction was performed by SN, with independent
verification of 50% of the entries by MA and MH to ensure accuracy and
consistency.
Quality Assessment and Risk of Bias
Although this review did not exclude studies based on quality, a descriptive
appraisal was undertaken. The following tools were used:
·
Newcastle-Ottawa Scale (NOS) for
cohort and case-control studies
·
Cochrane Risk of Bias Tool (RoB 2) for RCTs
Two reviewers independently assessed the quality of included
studies. Risk of bias domains evaluated included:
·
Selection bias
·
Performance bias
·
Detection bias
·
Attrition bias
·
Reporting bias
·
Confounding and exposure
misclassification
High-risk studies were not excluded but were analyzed separately
when applicable. A sensitivity analysis was performed to evaluate the
robustness of the review findings when excluding studies rated as high risk of
bias.
Data Synthesis
Given the diversity in study designs, populations, drug
classifications, and outcome measures, a narrative synthesis approach was
adopted. Findings were grouped by antidiabetic drug class and type of liver
cancer outcome (e.g., incidence, survival, recurrence). Patterns of
associations, inconsistencies, and gaps in evidence were summarized
thematically. Quantitative synthesis (meta-analysis) was not feasible due to
substantial heterogeneity across included studies.
Ethical Considerations
As this study was a review of previously published data, no ethical
approval or informed consent was required.
Results
The review process details are depicted in
the PRISMA flowchart (Figure 1). A total of 1,097 records were identified through database searches, including
Science Direct (n=1,000), PubMed (n=83), and Mendeley (n=14). After removing 16 duplicate records, 1089 records remained for title and
abstract screening. Following this initial screening, 1,046 records were excluded based on irrelevance to the study
objectives. Subsequently, 43 full-text articles were assessed for eligibility.
Among these, 25 studies were
excluded due to reasons such as wrong study design (n=17), in vitro study
(n=7), and irrelevant outcome (n=1). Ultimately, 18 studies met the inclusion
criteria and were included in the systematic review for further in-depth
analysis on the core relationship between antidiabetic drugs and the risk of
developing liver cancer.
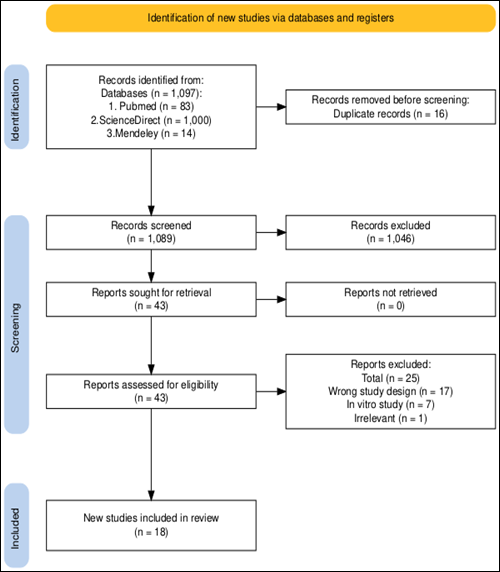
Figure 1. Prisma flow
diagram illustrating the study selection process.
This flowchart
illustrates the PRISMA process used for identifying, screening, and including
studies in a systematic review. It shows the number of records retrieved from
databases, the removal of duplicates, the number of records screened and
excluded, and the final count of studies included in the review (n = 18). The
diagram also details reasons for exclusion at each stage.
Participants and study characteristics
This systematic review included 18 studies,
encompassing a total of 3,572,638 participants across multiple geographic
regions (Table 1), including the USA, Taiwan, South Korea, China, Japan, Italy,
the Netherlands, and Australia. Among them, the USA contributed the highest
number of studies with 5, followed by Taiwan with 4, and China, Japan, and
Italy with 2 each. Several other countries, including South Korea, the
Netherlands, and Australia, each contributed 1 study.
This distribution highlights a significant
concentration of studies in the USA and East Asian countries, reflecting a
diverse geographic spread of research (Figure 2).
However, it is important to consider that
regional differences in diabetes prevalence, genetic predispositions,
healthcare infrastructure, and treatment protocols may have influenced the
outcomes observed. For instance, the pharmacogenomic response to antidiabetic
drugs and baseline liver cancer risk may vary between populations, potentially
limiting the generalizability of certain findings. Acknowledging these regional
disparities is essential when interpreting the data and applying conclusions
globally.
Table 1. Country distribution of
included studies.
|
Country
|
Count
|
|
USA
|
5
|
|
South Korea
|
1
|
|
Taiwan
|
4
|
|
China
|
2
|
|
Japan
|
2
|
|
Italy
|
2
|
|
Netherlands
|
1
|
|
Australia
|
1
|
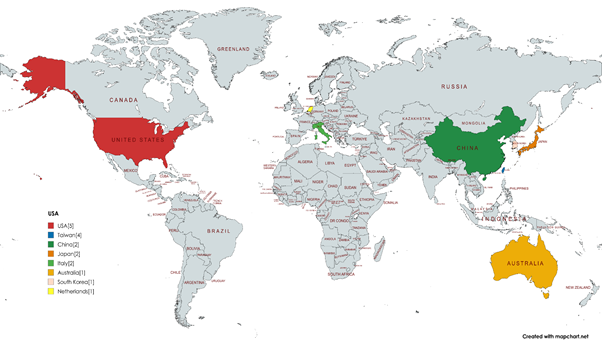
Figure 2. Country
distribution of studies. The image indicates the number of representations
by country, with each color corresponding to a
different country. The USA has the highest count (5), followed by Taiwan (4),
and several countries with 1–2 representations each, including China, Japan,
Italy, Australia, South Korea, and the Netherlands.
The majority of studies were retrospective
cohort studies (n=15), followed by observational (cross-sectional study) (n=2)
and multicenter retrospective (n=2). Additionally,
Comparative cohort, population-based case-control study, and population-based
cohort, as well as clinical and preclinical experimental studies, were
observed. Overall, the data reflect a dominance of retrospective cohort designs
with a mix of other observational and experimental methodologies.
Table 2. Methodological Designs of Included Studies.
|
Study Method
|
Count
|
|
Retrospective Cohort Studies
|
14
|
|
Population-based case-control study
|
1
|
|
Cross-sectional Studies
|
2
|
|
Population-based Clinical Transitional
study
|
1
|
Most studies utilized retrospective cohort
designs, particularly in the USA and Taiwan, while European and East Asian
studies incorporated cross-sectional, case-control, and clinical transitional
methodologies. The study populations varied significantly in size, ranging from
7 participants in an observational study to large-scale population-based
studies including 1 million individuals.
The age range of participants varied across
studies, with some reporting mean or median ages, while others provided
specific age brackets. The mean age of participants ranged from 15 to 80 years.
Certain studies distinguished between patients with and without cirrhosis,
reporting a higher mean age for cirrhotic patients. But some of the studies
didn’t mention any age-related data. Gender distribution was predominantly
mixed, although 2 studies focused on male participants. (Table 3).
Table 3. Key
characteristics of studies included in the systematic review.
|
Study Reference
|
Country
|
Study Design
|
Number of Participants
|
Age
|
Gender
|
Limitations of Each Study
|
|
(1)
|
USA
|
Retrospective
cohort
|
16,058
|
Mean age: For
patients without cirrhosis, 60.56 years (standard deviation [SD] = 10.31
years).
For patients with cirrhosis 66.99
years (SD = 7.09 years).
|
Predominantly
male
|
1. Unmeasured
Confounding Factors and Diagnosis Misclassification, 2. Short
Follow-Up Duration, 3. Limited Generalizability, 4. Unvalidated
Definition of Decompensated Cirrhosis
|
|
(2)
|
USA
|
Retrospective cohort
|
137,863
|
Median age: 62 years for metformin
users and 67 years for sulfonylurea users.
|
Both
|
1.
Unmeasured Confounding Factors, 2.
Diabetes Duration Not Considered, 3.
Insulin Effects Not Examined, 4.
Limited Generalizability, 5. Methodological Flaws in Prior Studies
|
|
(3)
|
USA
|
Retrospective
cohort
|
1,890,020
|
Mean age:
56.2 years
|
Both
|
1.
Retrospective Observational Design, 2.
Potential Unmeasured Confounding Factors, 3. Limited Follow-Up Duration, 4.
Generalizability to Non-Veteran Populations, 5. Methodological Limitations in
Prior Studies
|
|
(4)
|
South Korea
|
Comparative cohort
|
201,542
|
>45
|
Both
|
1.
Retrospective Design, 2. Missing Patient Details, 3. Limited Generalizability,
4. Potential Biases, 5. Uncertain Etiology
|
|
(5)
|
USA
|
Retrospective
cohort
|
23926
|
>50
|
Both
|
1.
Observational Study Design, 2. Unmeasured Confounders, 3. Data Limitations
|
|
(6)
|
Taiwan
|
retrospective cohort
|
36,853
|
Mean: 55.09
|
Both
|
1. ICD-10 Code Limitations, 2.
Small Sample Sizes in Minority Groups, 3. Need for Comprehensive Research
|
|
(7)
|
USA
|
retrospective
cohort
|
3,185
|
Mean: 74.8
|
Both
|
1. Ethnic
Specificity, 2. Unaccounted Lifestyle Factors, 3. Insulin Therapy Effects, 4.
Data Management Challenges, 5. Lifestyle Factors and Health Risks, 6. Need
for Comprehensive Research
|
|
(8)
|
China
|
retrospective analysis
|
159
|
Mean:56
|
|
1. Shifts in Diabetes Treatments
Over Time, 2. Unaccounted Health
Behaviors, 3. Lack of Treatment Classification
Data, 4. Retrospective Design, 5. Sample Size and Diversity, 6. Long-Term Effects of Metformin, 7. Interactions with Other Treatments
|
|
(9)
|
Netherlands
|
Population
based cohort
|
207,367
|
Median age:
61
|
Both
|
1. Misclassification
of NAFLD Diagnoses, 2. Lack of
Detailed Lifestyle Data, 3. Small
Sample Sizes
|
|
(10)
|
Japan
|
Observational (cross sectional
study)
|
7
|
Not specified
|
Not specified
|
1.
Small Sample Size, 2. Reliance on Liver Biopsies, 3. Potential Bias from Pharmaceutical Funding,
4. Limited Validation of GLP-1R
Expression
|
|
(11)
|
Taiwan
|
Population
based case control study
|
47,160
|
Mean age:
65.3
|
Both
|
1. Case-Control Design Limitations, 2. Absence of Lifestyle Data, 3. Sample Size Concerns
|
|
(12)
|
Italy
|
Clinical transitional study
|
70
|
28~89
|
Both
|
1.
In Vitro Model Limitations, 2.
Heterogeneity of HCC, 3.
Metformin's Long-Term Effects, 4.
Patient Variability, 5. Need
for Further Research
|
|
(13)
|
Italy
|
Multicenter
retrospective
|
279
|
N/A
|
N/A
|
1. Retrospective Data Bias, 2. Heterogeneity of HCC Patients, 3. Impact of Metabolic Status and
Comorbidities, 4. Need for Prospective
Trials
|
|
(14)
|
Taiwan
|
Retrospective cohort
|
1000000
|
40~60
|
Both
|
1.
Observational Study Design, 2.
Genetic Variability and Unmeasured Confounders
|
|
(15)
|
Australia
|
Retrospective
cohort
|
299
|
40~60
|
Both
|
1. Observational Study Design, 2. Uncontrolled Confounding Factors
|
|
(16)
|
Taiwan
|
Multi center cohort
|
7249
|
Older
|
Male
|
1.
Observational Study Design, 2. Uncontrolled
Confounding Factors
|
|
(17)
|
China
|
Retrospective
|
123
|
15~75
|
Both
|
1. Observational Study Design, 2. Confounding Factors
|
|
(18)
|
Japan
|
Cross sectional
|
478
|
40~80
|
Both
|
1.Observational Study Limitations,
2.Bias and Confounding, 3.Variations
in Patient Care, 4.Sample Size and Patient
Heterogeneity
|
Risk of bias assessment
A systematic risk of bias assessment was
conducted for the 18 studies included in this review, evaluating six key
domains: selection of participants, confounding variables, measurement of
exposure, blinding of outcome assessment, incomplete outcome data, and
selective outcome reporting (Figure 2).
Among the studies, 15 were classified as
high risk and 3 as low risk for participant selection, indicating a substantial
risk in this domain. Confounding variables were a concern, with 5 studies at
high risk, 5 at low risk, and 8 marked as unclear, reflecting variability in
controlling confounders. Measurement of exposure showed 7 studies at high risk,
6 at low risk, and 5 as unclear, suggesting inconsistencies in exposure
assessment. A significant limitation was blinding of outcome assessment, with
11 studies marked as unclear and 7 as high risk, highlighting a lack of
transparency in blinding procedures. Incomplete outcome data were generally
well managed, with 13 studies classified as low risk, 4 as high risk, and 1 as
unclear, ensuring comprehensive reporting in most cases. Similarly, selective
outcome reporting was well handled, with 15 studies assessed as low risk and 3
as high risk, indicating minimal bias in this area. Overall, while certain
domains, such as blinding and participant selection, posed a high risk of bias,
the handling of outcome data and selective reporting was relatively robust.
Association between anti-diabetic drugs and liver cancer
Key findings of the included studies are
given below (Table 4) according to the different classes of anti-diabetic
drugs.
Recent studies have illuminated the
intricate relationship between antidiabetic therapies and outcomes related to
hepatocellular carcinoma (HCC) in patients with type 2 diabetes.
Thiazolidinediones have shown a strong negative association with HCC risk, indicating
a potential protective effect (9). Similarly, sodium-glucose cotransporter 2 (SGLT2)
inhibitors have been linked to significantly lower mortality risks in HCC
patients, especially those with chronic viral hepatitis (7). Additionally, GLP-1 receptor agonists are
associated with a reduced risk of HCC and hepatic decompensation, suggesting
that their benefits extend beyond diabetes management (1). Conversely, insulin use in individuals with type
2 diabetes and cirrhosis has been associated with an increased risk of all-cause
mortality and severe complications, highlighting the need for vigilant
management in this high-risk group redu(5). Furthermore, metformin therapy has demonstrated
promising outcomes, correlating with improved survival rates in patients with
biopsy-proven nonalcoholic steatohepatitis (NASH) and
compensated cirrhosis, particularly in those with elevated HbA1c levels (15). The combination of metformin and SGLT2
inhibitors warrants further exploration, as their synergistic effects could
enhance patient outcomes (18). While DPP-4 inhibitors suggest a potential
protective effect against HCC, existing studies are primarily observational,
necessitating additional research to establish causative relationships (14). Ultimately, these findings advocate for a
personalized
diabetes management approach that
prioritizes hepatic health, aiming to improve outcomes for at-risk populations.
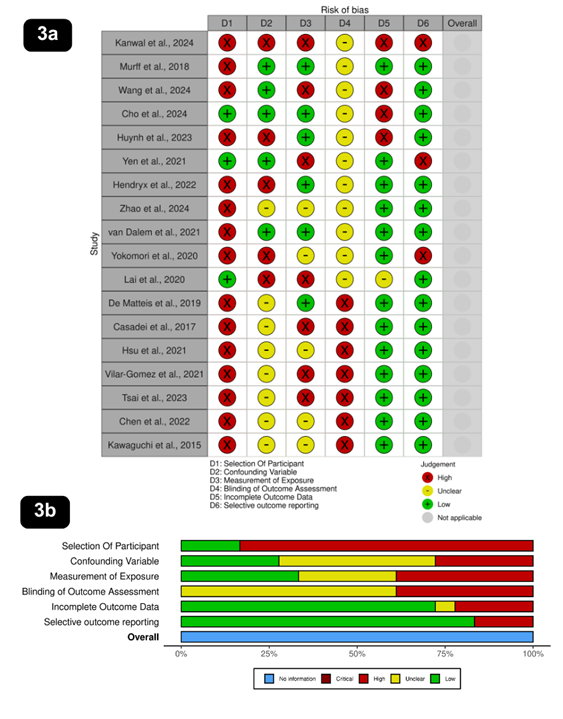
Figure 3. Risk of bias assessment across included studies. Figure 3a shows
the proportion of studies assessed for various domains of bias, including:
selection of participants, confounding variables, measurement of exposure,
blinding of outcome assessment, incomplete outcome data, and selective outcome
reporting. Each domain is color-coded to represent the assessed level of bias:
Low risk (green), Unclear risk (yellow), High risk (red), Critical risk (dark
red), and No information (blue). Figure 3b (see image below) provides a
study-wise breakdown of risk of bias assessments, allowing a granular
comparison across individual studies.
These assessments were conducted using the ROBINS-I tool (Risk Of Bias In Non-randomised
Studies - of Interventions), which is designed to evaluate the risk of bias in
non-randomized intervention studies. The visual summaries aid in identifying
methodological limitations and potential biases that may influence the
reliability of the reported outcomes (1-18).
Table 4. Association between
Antidiabetic drugs and liver cancer.
|
Drug/Management
|
Findings
|
References
|
|
Thiazolidinediones
|
- Associated with a negative correlation between their use and
the risk of developing HCC in type 2 diabetes patients.
- For each additional year of use, a lower risk of HCC was
observed.
- HR 0.67, 95% CI: 0.53–0.84 for each additional year of use.
|
(11)
|
|
Sodium-Glucose
Cotransporter 2 (SGLT2) Inhibitors
|
- The initiation of SGLT2 inhibitors in patients with HCC
resulted in lower mortality risk.
- Longer duration of SGLT2 use was linked to greater survival
benefits.
- Notably beneficial in patients with chronic viral hepatitis.
- Adjusted HR 0.72, 95% CI: 0.60–0.86.
|
(7)
|
|
GLP-1
Receptor Agonists
|
- Linked to a significantly reduced risk of developing HCC in
type 2 diabetes patients.
- May help in preventing hepatic decompensation in this
population.
- Suggests protective mechanisms against liver complications.
- HR 0.58, 95% CI: 0.45–0.75.
|
(1)
|
|
Metformin
|
- Associated with improved survival outcomes in patients with biopsy-proven
NASH and compensated cirrhosis.
- Significant reduction in risks of death and hepatic
complications.
- Particularly effective in patients with HbA1c levels above
7.0%.
- RR 0.65, 95% CI: 0.50–0.84; especially effective with HbA1c
>7.0%.
|
(15)
|
|
Insulin
(in Type 2 Diabetes with Cirrhosis)
|
- Associated with a higher risk of all-cause mortality and
liver-related complications.
- Increased prevalence of severe hypoglycemia and cardiovascular
events observed.
- Indicates a need for careful treatment consideration and
monitoring.
- HR 1.42, 95% CI: 1.18–1.70.
|
(5)
|
|
Dual
Therapy (Metformin and SGLT2i)
|
- Suggested for future studies to validate the efficacy in larger
and more diverse populations.
- Potential for improving treatment outcomes when tailored to
individual patient needs.
- Emphasizes comprehensive patient assessments.
|
(4)
|
|
DPP-4
Inhibitors
|
- Observed potential protective effects against HCC.
- Causal relationships remain to be established due to the
observational nature of current studies.
- Further research is needed to confirm findings and explore
underlying mechanisms.
- Reported HR ranges from 0.70 to 0.85 in subgroup analyses.
|
(1)
|
Influencing factors for hepatocellular carcinoma due to diabetes management
The summary below emphasizes the factors
influencing hepatocellular carcinoma (HCC) within the framework of diabetes
management. Key determinants include patient demographics, such as age, gender,
and general health status, which notably affect clinical outcomes (14).
Additionally, comorbid conditions like chronic liver diseases—specifically
cirrhosis and non-alcoholic fatty liver disease—have been identified as
contributing to the elevated risk of HCC (13, 27). The therapeutic impact of
antidiabetic medications, including metformin and SGLT2 inhibitors,
demonstrates potential benefits in improving patient prognosis and reducing
mortality associated with HCC (14, 26). Moreover, lifestyle factors,
including physical activity and dietary habits, play a crucial role in
augmenting the effectiveness of treatment regimens and influencing disease
progression (17, 18). Additionally, the selection of therapeutic interventions,
such as transarterial chemoembolization and its
integration with other treatment modalities, has been shown to significantly
impact patient survival (26). Collectively, these findings underscore the
necessity for personalized treatment strategies and the importance of closely
monitoring metabolic health to diminish the risk of HCC among individuals with
diabetes (14, 27).
Discussion
The relationship between anti-diabetic
drugs and liver cancer, particularly hepatocellular carcinoma (HCC), has gained
significant attention in recent years. Our systematic review provides a
thorough analysis of the relationship between anti-diabetic drugs and liver
cancer risk, progression, complications, treatment outcomes, and prognosis. The
studies included in our review originated from diverse geographic locations,
with the USA (5) and Taiwan (4) contributing the most research. Additionally,
this review integrates evidence from various study designs, including
retrospective cohort studies, population-based analyses, and preclinical
experimental research that provide insights into the complex interplay between
diabetes management and liver cancer risks.
Among the anti-diabetic medications
reviewed, Metformin consistently shows the strongest protective effect against
HCC, beyond its role in blood sugar control. Patients with type 2 diabetes and
cirrhosis treated with metformin exhibit a significant 56% reduction in HCC risk compared to those using sulfonylureas (2). Furthermore, metformin serves a protective role
in reducing the risk of hepatic decompensation and death in type 2 diabetic
patients with HbA1c >7.0% (15) Metformin provides long-term liver protection and
reduces complications in type 2 diabetic patients with chronic hepatitis C
(CHC) who achieved sustained virological response (SVR) after antiviral therapy
(16). Metformin exerts its beneficial effects through
modulation of metabolic pathways. For example, It
enhances AMP-activated protein kinase (AMPK) that decreases metabolic and
survival pathways of tumor cells. It also inhibits
mTOR, which suppresses tumor growth pathways and
upregulates SIRT3, which improves mitochondrial function and reduces oxidative
stress. Additionally, the reduction of HIF-1α limits hypoxia-driven tumor progression. Ultimately, these mechanisms lead to
decreased tumor cell proliferation (12,13).
A separate study found that low-dose
metformin inhibits HCC cell migration by reducing interleukin-8 (IL-8)
secretion, which reduces
inflammation and plays a role in tumor progression
and metastasis. This suggests that metformin might help slow cancer spread, potentially improving prognosis and quality of life for HCC patients (8). Additionally, metformin has shown enhanced
progression-free survival (PFS) and overall survival (OS) rates in patients
taking Sorafenib, a tyrosine kinase
inhibitor (TKI) used as targeted
therapy for advanced HCC, suggesting its potential in both prevention and
adjunctive cancer therapy (13). A study found that trans-arterial
chemoembolization (TACE) combined with metformin improves HCC prognosis in type
2 diabetes patients, enhancing treatment efficacy and survival (17).
Similarly, thiazolidinediones (TZDs) are
strongly associated with reduced risk of non-alcoholic fatty liver disease
(NAFLD) and HCC (9,11). Thiazolidinediones (TZDs), such as pioglitazone
and rosiglitazone, with longer duration of use, may reduce the risk of
hepatocellular carcinoma (HCC) through multiple mechanisms, primarily by
improving insulin resistance, reducing inflammation, and exerting direct anti-tumor effects (11). It activates peroxisome proliferator-activated
receptor gamma (PPAR-γ), which
enhances insulin sensitivity in peripheral tissues and reduces
hyperinsulinemia, which decreases hepatocyte proliferation and carcinogenesis (19).
GLP-1 receptor agonists have potential in
reducing hepatocellular carcinoma (HCC) risks and hepatic decompensation
despite their well-known benefits in glycemic control
and cardiovascular protection, adding another dimension to their clinical
significance (3). A study also highlights the significant role of
GLP-1 receptor agonists in reducing the risk of cirrhosis and HCC, particularly
in patients with metabolic dysfunction-associated steatotic
liver disease (MASLD) (1). These drugs modulate fat metabolism, reduce fat
in liver parenchyma, and decline the progression of fibrosis, have
anti-inflammatory effects, and exert hepatic protection (1,9).
The long-term use of DPP-4 inhibitors
lowers the risk of hepatocellular carcinoma (HCC) in patients with type 2
diabetes and chronic HCV infection by preventing CXCL10 truncation that
diminishes HCV viral load and enhances immune response. So, it could indeed be
a valuable second-line therapy after metformin for patients with both diabetes
and chronic HCV (14). But a study shows GLP-1 receptor agonists are
superior in terms of reducing cirrhosis progression and related complications
compared to DPP-4 inhibitors (1).
In contrast, studies based on SGLT2
inhibitors (SGLT2i) reveal a bit of controversial information. One study shows
that longer duration of SGLT2 inhibitor use is associated with greater survival
benefits through mechanisms like reducing
systemic inflammation, improving
metabolic control, and potentially
limiting liver fibrosis or tumor progression (7). Again, the combination of metformin and SGLT2
inhibitor appears to have a beneficial effect in reducing complications and
morbidity in HCC (5). On the other hand, another study reveals that
SGLT2 inhibitors did not significantly reduce HCC risk in patients with NAFLD
and T2DM. But the benefit is population dependent because SGLT2 inhibitors have
a promising positive effect in HCC risk reduction in patients with HCV
infection (4).Although insulin therapy is effective in
controlling blood glucose, its use in cirrhotic patients may lead to adverse
outcomes like higher mortality, liver complications, cardiovascular events, and
hypoglycemia. As hyperinsulinemia worsens the
condition of cirrhosis and HCC, administration of exogenous insulin in type 2
diabetic patients who have more or less insulin resistance results in a
profound increase in blood insulin level that deteriorates the metabolic
condition of the liver and enhances tumorigenesis (6,13). Therefore, instead of insulin therapy, other
medications such as metformin, Thiazolidinediones or GLP-1 receptor agonists
are efficacious to reduce the risk of NAFLD, cirrhosis, and HCC (9,13).
Key recommendations
Recommendations from the included studies
with their key insights are given in the table below (Table 5).
Despite potential evidence pointing towards
a possible association between antidiabetic drugs and the risk of liver cancer,
the inconclusiveness of available data emphasizes the need for further
comprehensive research. Future studies should consider larger and more diverse
study populations to validate the generalizability of findings. Randomized
controlled trials (RCTs) and prospective cohort studies should be prioritized
to confirm the causal relationships between antidiabetic drugs and HCC risk
reduction. Again, mechanistic studies can be done further to delve into the biological, molecular, or
physiological mechanisms of how different antidiabetic drugs influence liver
cancer development.
For high-risk cirrhotic patients where
insulin is associated with increased mortality and hepatic complications,
alternative management strategies should be explored (20). These may include the cautious use of metformin
(with liver function monitoring), SGLT2 inhibitors, and GLP-1 receptor
agonists—agents that have demonstrated potential hepatic benefits in
non-cirrhotic populations (21). In patients with compensated cirrhosis,
individualized treatment regimens and close monitoring of glycemic
control, liver enzymes, and nutritional status are essential (20). Consultation with hepatologists and
endocrinologists is also advised for optimizing therapy in this complex patient
group.
Table 5. Key
recommendations of selected studies.
|
References
|
Recommendations
|
Key insights
|
|
(5)
|
Future
research should involve larger and more diverse populations to validate the
benefits of dual therapy; explore the long-term safety of treatments.
|
To enhance
the applicability and robustness of findings across different demographic
groups.
|
|
(9)
|
Future
studies should adopt prospective designs to understand the long-term effects
of TZDs on liver health; include lifestyle factors in analyses.
|
To achieve
a nuanced understanding of TZD effects and improve treatment strategies for
patients.
|
|
(3)
|
Future
research should focus on prospective trials to explore the long-term effects
of GLP-1 receptor agonists; consider diverse patient populations.
|
To
validate effectiveness and explore the mechanisms behind GLP-1 RAs in various
demographics.
|
|
(1)
|
Highlights
the significance of early treatment with GLP-1 receptor agonists and
encourages future research on long-term outcomes across varied populations.
|
To promote
early intervention strategies to improve liver health outcomes among at-risk
patients.
|
|
(11)
|
Conduct
further studies to explore the protective effects of TZDs against liver
cancer; include lifestyle factors in future analyses.
|
To
establish a causal relationship and better understand the protective role of
TZDs in liver cancer risk reduction.
|
|
(15)
|
Emphasizes
the need for randomized trials to clarify metformin's role; monitor patients
for lifestyle changes.
|
To
establish clear causal links and improve management strategies for
diabetes-related liver conditions.
|
Further works should highlight the
following points-
1. Larger and Diverse Studies: Future
research should involve larger and more diverse populations to enhance the
generalizability of findings, particularly regarding the efficacy of metformin
and SGLT2 inhibitors in improving liver health outcomes (5).
2. Long-term Prospective Research: There is a need for prospective studies to investigate the long-term
effects of thiazolidinediones (TZDs) and their interactions with lifestyle
factors to better understand their role in liver health (9).
3. Evaluation of GLP-1 Agonists: Research should focus on the long-term effects of GLP-1 receptor
agonists across various demographics to confirm their protective benefits
against liver complications (3).
4. Early Intervention: Emphasizing early treatment with GLP-1
receptor agonists for at-risk patients can significantly improve liver health
outcomes (1).
5. Establish Causal Links for TZDs: More studies are needed to explore the protective effects of TZDs
against liver cancer, aiming to establish clear causal relationships (11).
6. Randomized Trials for Metformin: Conducting randomized controlled trials will help clarify metformin's
protective role in enhancing liver-related outcomes in patients with diabetes (15).
Clinical Implications
The findings from this review underline the
importance of incorporating antidiabetic medications such as metformin, TZDs,
and GLP-1 receptor agonists into diabetes management, not only for glycemic control but also for their potential to reduce the
risk of liver cancer (22–24). These medications, particularly metformin and
GLP-1 receptor agonists, demonstrate significant protective effects against
hepatocellular carcinoma (HCC), highlighting their dual benefits in both
diabetes management and liver health (25,26). Early intervention with GLP-1 receptor
agonists, especially in at-risk populations, may offer substantial long-term
benefits, potentially reducing the risk of hepatic decompensation and HCC
progression (26,27). Moreover, individualized treatment strategies
based on patient demographics, liver disease severity, and metabolic factors
are essential for optimizing therapeutic outcomes (28). In high-risk patients, such as those with
cirrhosis, careful selection of medications and close monitoring are critical (29). These insights emphasize the need for
personalized, patient-centered approaches that
incorporate both metabolic and genetic factors to enhance treatment efficacy,
minimize adverse effects, and improve overall patient outcomes, particularly in
preventing liver cancer among individuals with diabetes.
Limitations and future directions
This systematic review, while thorough, has
several limitations. Firstly, many of the included studies were observational,
limiting the ability to establish causality between antidiabetic medication use
and liver cancer outcomes. Observational studies are prone to various biases,
such as selection and information bias, which may affect the reliability of the
findings.
Secondly, due to restricted access to
certain databases, we were unable to include relevant studies from platforms
like Google Scholar, potentially missing important research.
Thirdly, the review primarily focused on
observational studies and did not include systematic reviews or meta-analyses,
which could have provided a broader perspective on the topic.
Additionally, small sample sizes in some
studies limited the generalizability of the results and increased the risk of
type II errors.
Lastly, several studies did not adequately
control for confounding factors, such as patient demographics, comorbidities,
and concurrent treatments, which may have impacted the results. The variability
in study designs, drug types, dosages, and follow-up periods also introduced
significant heterogeneity, complicating the synthesis of findings and making
definitive conclusions difficult.
Conclusion
This study underscores the complex and
significant relationship between antidiabetic therapies and hepatocellular
carcinoma (HCC) outcomes in patients with type 2 diabetes. Evidence suggests
that certain antidiabetic medications, such as thiazolidinediones (TZDs),
sodium-glucose cotransporter 2 (SGLT2) inhibitors, and GLP-1 receptor agonists,
show promising protective effects against HCC and liver-related complications.
Conversely, insulin use in patients with cirrhosis appears to increase the risk
of mortality and severe complications, highlighting the need for cautious
management in this high-risk group.
Additionally, metformin demonstrates
potential benefits, particularly in patients with non-alcoholic steatohepatitis
(NASH) and compensated cirrhosis. However, future research should prioritize
long-term randomized controlled trials (RCTs), well-designed population-based
cohort studies, and mechanistic studies to better validate and clarify the
protective effects of antidiabetic therapies across various liver disease
contexts. Such studies should also assess treatment duration, dosage,
and interactions with coexisting conditions to optimize diabetes management
strategies in patients at risk for liver cancer.
This review also emphasizes the importance
of a personalized treatment approach that takes into account both pharmacologic
therapies and lifestyle factors, aiming to reduce the risk of HCC in
individuals with diabetes. Close monitoring of metabolic health and early
intervention with appropriate medications can significantly improve outcomes in
at-risk populations.
In summary, while current evidence
highlights the potential of various antidiabetic therapies to positively
influence liver health and HCC outcomes, further investigation using rigorous
study designs is crucial to establish clearer causal relationships and refine
clinical strategies for managing diabetes in patients with liver disease.
Author contribution
SN developed the methodology and wrote the methodology section. SN
also conducted data extraction using a predesigned Excel spreadsheet, capturing
key study details, including study design, patient population, type of
antidiabetic medications used, liver cancer outcomes, and major findings.
Additionally, SN oversaw the entire review process and coordinated the writing
of the manuscript. MA independently verified 50% of the extracted data
to ensure accuracy and consistency. MA also wrote the results section,
contributed to the final review of the manuscript, played a role in developing
the study design, and assisted in refining the methodology section. SH contributed
to refining the search strategy, participated in the full-text review process,
and assisted in synthesizing the extracted data. SH also built the tables and diagrams
for the manuscript and helped review the methodology section. MSH independently
conducted the title and abstract screening using Rayyan software, ensuring the
initial selection of studies. MSH also conducted the full-text review for
studies meeting the inclusion criteria and wrote the discussion section. MH independently
verified 50% of the extracted data alongside MA to enhance data accuracy. MH
also contributed to refining the study methodology and participated in
manuscript revisions. AA wrote the introduction section and assisted in
optimizing the search strategy. AA also played a role in screening full-text
articles and contributed to drafting and reviewing the discussion section. TD
independently conducted the title and abstract screening using Rayyan
software, ensuring the initial selection of studies. TD also wrote the
conclusion section and participated in discussions regarding study inclusion
and exclusion criteria. SoN contributed to
writing the discussion section and provided critical revisions to improve
clarity and coherence. SoN also participated in
reviewing the final manuscript to ensure consistency and accuracy. AN played
a role in the quality assessment of included studies and assisted in
synthesizing the extracted data. AN also contributed to reviewing the
discussion and conclusion sections to ensure alignment with the study
objectives. All authors contributed to the conception and design of the
study, provided input on data interpretation, and participated in manuscript
revisions. All authors approved the final version before submission.
Conflict of interest
The author declares no conflict of interest associated with this
paper.
Funding
There is no funding.
References
1. Kanwal F, Kramer JR, Li
L, Yang YX, Cao Y, Yu X, et al. GLP-1 Receptor Agonists and Risk for Cirrhosis
and Related Complications in Patients With Metabolic
Dysfunction-Associated Steatotic Liver Disease. JAMA
Intern Med. 2024 Nov 1; 184(11):1314.
2. Murff HJ, Roumie CL, Greevy RA, Hackstadt
AJ, McGowan LEDa, Hung AM, et al. Metformin use and
incidence cancer risk: evidence for a selective protective effect against liver
cancer. Cancer Causes Control. 2018 Sep 1; 29(9):823–32.
3. Wang L, Berger NA,
Kaelber DC, Xu R. Association of GLP-1 Receptor Agonists and Hepatocellular
Carcinoma Incidence and Hepatic Decompensation in Patients With
Type 2 Diabetes. Gastroenterology. 2024 Sep 1; 167(4):689–703.
4. Cho HJ, Lee E, Kim SS,
Cheong JY. SGLT2i impact on HCC incidence in patients with fatty liver disease
and diabetes: a nation-wide cohort study in South Korea. Sci Rep. 2024 Apr 29;
14(1):9761.
5. Huynh DJ, Renelus BD, Jamorabo DS. Reduced mortality and morbidity associated
with metformin and SGLT2 inhibitor therapy in patients with type 2 diabetes
mellitus and cirrhosis. BMC Gastroenterol. 2023 Dec 1; 23(1).
6. Yen FS, Lai JN, Wei
JCC, Chiu LT, Hsu CC, Hou MC, et al. Is insulin the preferred treatment in
persons with type 2 diabetes and liver cirrhosis? BMC Gastroenterol. 2021 Dec
1; 21(1).
7. Hendryx M, Dong Y, Ndeke JM, Luo J. Sodium-glucose cotransporter 2 (SGLT2)
inhibitor initiation and hepatocellular carcinoma prognosis. PLoS One. 2022 Sep 1; 17(9).
8. Zhao C, Zheng L, Ma Y,
Zhang Y, Yue C, Gu F, et al. Low-dose metformin suppresses hepatocellular
carcinoma metastasis via the AMPK/JNK/IL-8 pathway. Int J Immunopathol
Pharmacol. 2024 Jan 1; 38.
9. van Dalem J, Driessen
JHM, Burden AM, Stehouwer CDA, Klungel OH, de Vries
F, et al. Thiazolidinediones and Glucagon-Like Peptide-1 Receptor Agonists and
the Risk of Nonalcoholic Fatty Liver Disease: A Cohort Study. Hepatology. 2021
Nov 1; 74(5):2467–77.
10. Yokomori
H, Ando W. Spatial expression of glucagon-like peptide 1 receptor and
caveolin-1 in hepatocytes with macrovesicular
steatosis in non-alcoholic steatohepatitis. BMJ Open Gastroenterol. 2020 May
14; 7(1).
11. Lai SW, Lin CL, Liao KF.
Association of hepatocellular carcinoma with thiazolidinediones use: A
population-based case-control study. Medicine. 2020 Apr 23 ;
99(17):E19833.
12. De Matteis S, Scarpi E, Granato AM, Vespasiani-Gentilucci
U, Barba G La, Foschi FG, et al. Role of SIRT-3, p-mTOR and HIF-1α in
Hepatocellular Carcinoma Patients Affected by Metabolic Dysfunctions and in
Chronic Treatment with Metformin. Int J Mol Sci. 2019 Mar 2; 20(6).
13. Casadei Gardini A, Faloppi L, De Matteis S, Foschi FG, Silvestris N, Tovoli F, et al. Metformin and insulin impact on clinical
outcome in patients with advanced hepatocellular carcinoma receiving sorafenib:
Validation study and biological rationale. Eur J
Cancer. 2017 Nov 1; 86:106–14.
14. Hsu WH, Sue SP, Liang
HL, Tseng CW, Lin HC, Wen WL, et al. Dipeptidyl Peptidase 4 Inhibitors Decrease
the Risk of Hepatocellular Carcinoma in Patients With
Chronic Hepatitis C Infection and Type 2 Diabetes Mellitus: A Nationwide Study
in Taiwan. Front Public Health. 2021 Sep 17; 9.
15. Vilar-Gomez E,
Calzadilla-Bertot L, Wong VWS, Castellanos M, Aller-de la Fuente R, Eslam M, et
al. Type 2 Diabetes and Metformin Use Associate With
Outcomes of Patients With Nonalcoholic
Steatohepatitis–Related, Child–Pugh A Cirrhosis. Clinical Gastroenterology and
Hepatology. 2021 Jan 1; 19(1):136-145.
16. Tsai PC, Kuo HT, Hung
CH, Tseng KC, Lai HC, Peng CY, et al. Metformin reduces hepatocellular
carcinoma incidence after successful antiviral therapy in patients with
diabetes and chronic hepatitis C in Taiwan. J Hepatol. 2023 Feb 1;
78(2):281–92.
17. Chen ML, Wu CX, Zhang
JB, Zhang H, Sun YD, Tian SL, et al. Transarterial
chemoembolization combined with metformin improves the prognosis of
hepatocellular carcinoma patients with type 2 diabetes. Front Endocrinol
(Lausanne). 2022 Sep 15; 13.
18. Kawaguchi T, Kohjima M, Ichikawa T, Seike M,
Ide Y, Mizuta T, et al. The morbidity and associated risk factors of cancer in
chronic liver disease patients with diabetes mellitus: a multicenter field
survey. J Gastroenterol. 2015 Mar 1; 50(3):333–41.
19. Vella V, Nicolosi ML,
Giuliano S, Bellomo M, Belfiore A, Malaguarnera R.
PPAR-γ agonists as antineoplastic agents in cancers with dysregulated IGF
axis. Front Endocrinol (Lausanne). 2017 Feb 22; 8(FEB):244472.
20. Puri P, Kotwal N. An
Approach to the Management of Diabetes Mellitus in Cirrhosis: A Primer for the
Hepatologist. J Clin Exp Hepatol. 2021 Mar 1; 12(2):560.
21. Padda IS, Mahtani AU,
Parmar M. Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors. StatPearls. 2023 Jun 3.
22. Weinberg Sibony R, Segev
O, Dor S, Raz I. Drug Therapies for Diabetes. Int J Mol Sci. 2023 Dec 1;
24(24):17147.
23. Arvanitakis K, Koufakis T, Kalopitas G,
Papadakos SP, Kotsa K, Germanidis
G. Management of type 2 diabetes in patients with compensated liver cirrhosis:
Short of evidence, plenty of potential. Diabetes & Metabolic Syndrome:
Clinical Research & Reviews. 2024 Jan 1; 18(1):102935.
24. Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the
treatment of type 2 diabetes – state-of-the-art. Mol Metab.
2020 Apr 1; 46:101102.
25. Huynh DJ, Renelus BD, Jamorabo DS. Dual metformin and glucagon-like peptide-1
receptor agonist therapy reduces mortality and hepatic complications in
cirrhotic patients with diabetes mellitus. Ann Gastroenterol. 2023 Aug 30;
36(5):555.
26. Shabil
M, Khatib MN, Ballal S, Bansal P, Tomar BS, Ashraf A, et al. Risk of
Hepatocellular Carcinoma with Glucagon-like Peptide-1 receptor agonist
treatment in patients: a systematic review and meta-analysis. BMC Endocr Disord. 2024 Dec 1;
24(1):246.
27. Wang L, Berger NA,
Kaelber DC, Xu R. Association of GLP-1 Receptor Agonists and Hepatocellular
Carcinoma Incidence and Hepatic Decompensation in Patients With
Type 2 Diabetes. Gastroenterology. 2024 Sep 1; 167(4):689–703.
28. Wazir H, Abid M, Essani B, Saeed H, Khan MA, Nasrullah F, et al. Diagnosis
and Treatment of Liver Disease: Current Trends and Future Directions. Cureus.
2023 Dec 4; 15(12):e49920.
29. Chandok N, Watt KDS.
Pain Management in the Cirrhotic Patient: The Clinical Challenge. Mayo Clin
Proc. 2010; 85(5):451.



