Introduction
Lung cancer
is the most common cause of cancer deaths in men, with non-small cell lung
cancer (NSCLC) being most common. About 30-40% of NSCLC patients present with
metastatic disease at the time of diagnosis (1).† Bone is the most common metastatic site,
followed by the lungs, brain, liver and adrenal glands. Brain metastasis is
considered to be an unfavorable prognostic factor. Palliation of symptoms
and preservation of neurologic function are the main goals of treatment for
many patients with brain metastases. Survival has improved by inclusion of MRI
brain in early detection. Whole brain radiotherapy alone or Stereotactic
radiosurgery/radiotherapy alone or in combination can be considered in brain
metastasis. Stereotactic radiosurgery is a non-surgical radiation therapy that
aims to deliver precisely targeted radiation in fewer high-dose treatments than
traditional therapy (2, 3). The size and location of lesions, the proximity of
organs at risk (OARs), and the biologically effective dose (BED) of each treatment
plan all influence the SRT dose and fractionation schemes. Multiple factors
influence the decision on SRS vs FSRS or WBRT. Graded prognostic assessment
(GPA) score, recursive partitioning analysis (RPA) class, synchronous or
metachronous diagnosis of Brain metastasis, and specifically volume > 10 cc
influences decisions. Similarly age, sex and tumor histology have been analyzed
for survival (4).
Current National Cancer Care Network (NCCN) guidelines recommend the use
of volume instead of the absolute number of metastases as the limit to
determine eligibility for SRS, with potential cutoffs being ≤15 cc.
Yamamoto et al.'s 2014 multi-institutional prospective observational analysis
showed no difference in OS or treatment-related adverse events between treating
2-4 brain lesions and 5-10 lesions (total volume 6 cm are not treated with SRS
(5). The Radiation Therapy Oncology Group Trial developed dose limits for SRS
of 24 Gy for lesions less than 2 cm, 18 Gy for lesions 2 to 3 cm and 15 Gy for tumors 3 to 4 cm. We used 24Gy in 3 fractions as
prescription dose.
Additionally, SRT calls for extra caution when it comes to prescribing,
documenting, and reporting. The potential advantages of SRS, include, its quick
treatment duration and high likelihood of treated-lesion control. Here we
report a case of metastatic lung carcinoma with multiple brain metastasis
treated with single isocenter multiple target
stereotactic fractionated radiotherapy at our institute.
Case presentation
A 62-year-old male previously treated for stage IIIC Adenocarcinoma
elsewhere with chemotherapy presented to us with severe and persistent
headache, worsening over time since 6 months. The
headache was more in the morning and was associated with nausea and vomiting.
Associated other symptoms include blurring of vision. Neurological examination
was within normal limits except for vision impairment. He underwent Contrast
enhanced magnetic resonance imaging (CEMRI) of brain in view of suspected brain
metastasis as radiological imaging to evaluate the symptoms. Disease mapping
with PET CT was suggestive of enhancing irregular margined left infrahilar and suprahilar mass
measuring 5.8 x 6.6 cm (SUV max 18.18). Biopsy from Hilar growth was suggestive
of non-small cell Lung Carcinoma. Immunohistochemistry results were positive
for CK7 and negative for P63, P40, CK20, Synatophysin,
chromogranin, CD56 and TTF-1. Overall favoring as poorly Differentiated
Adenocarcinoma. He was prescribed dexamethasone at 16 mg /day in divided doses
along with tab emset 4 mg thrice daily, tab pantop 40mg once daily and pain medications to relieve the
symptoms.
Radiological Imaging:
Contrast enhanced MRI (CEMRI) of the brain revealed multiple
intracranial brain lesions suggestive of metastasis. An irregular thick walled
conglomerated peripherally enhancing lesion involving the right occipital lobe
measuring 18 x 16 mm, another lesion in the left parietal lobe measuring 17 x
19 mm at the gray white junction with surrounding parietal edema and a tiny
ring enhancing intra- axial lesion measuring 5 x 0.7 x 5 mm in the left high
parietal lobe with minimal surrounding edema (Figure 1). These radiological and
pathological findings were consistent with multiple brain metastases in a
previously treated case of pulmonary adenocarcinoma.
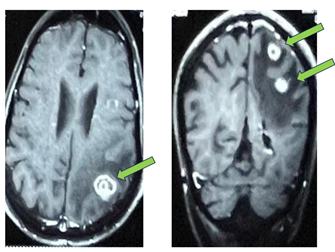
Figure 1. CEMRI Brain showing multiple brain metastasis.
He was discussed in multidisciplinary committee composed of
neurosurgeon, radiation oncologist, and medical oncologist, and was planned
with fractionated stereotactic radiosurgery (FSRS). He was planned with single
isocenter multiple target FSRS using 3 Planning target volumes (PTV), each
against the gross tumor volume (GTV) (enhancing lesions in right occipital
lobe, left parietal lobe and high parietal lobe). In the present case, there
were multiple, 3 brain metastasis of diameter 2.2 cm, 1.8 cm and 1.6 cm, the
radiation tumor board decided for multi fraction SRS with 24 Gy marginal dose
as per RTOG 9005. Multiple plans were generated, and best plan was delivered
post stringent and recommended dosimetry and quality check by medical
dosimetrist.
Discussion
The concept of
SRS was introduced by Larks Leksell in 1951 as an alternative treatment option
to conventional WBRT (2). It can be delivered with the help of Gamma knife
which uses 192 small beams of gamma rays or with LINAC which uses X-rays
(photons) to target and treat cancerous (Gliomas, brain metastasis,
meningiomas, vestibular schwannomas) and noncancerous brain abnormalities
(vascular pathologies, and functional disorders) (6). Charged particle
radiosurgery or Proton therapy is relatively new and is available at very few
centers. SRS Delivery is done accurately within 1-2 mm. When given in two or
more fractions, it is termed as fractionated radiotherapy (3).
Immobilization: The
radiotherapy procedure involves frameless immobilization, imaging, dose
planning and radiation delivery after quality assurance. We used Encompass SRS
immobilization System. It provides noninvasive stereotactic immobilization by
using a patient-specific thermoplastic mask. It is designed for precisely
targeting brain treatments. Furthermore, it conforms to patient features to
provide accurate, reproducible positioning, repositioning and immobilization.
Likewise, it also allows for diagnostic imaging in the same position. The mask
features the Integra Bite, which reduces motion, allowing for maximum dose to
the tumor and minimizing radiation delivered to the surrounding healthy tissue.
The mask utilizes a posterior thermoplastic and anterior open view for use with
an optical tracking system to allow for real-time monitoring. Encompass insert
attaches to KVue Couch top and K Vue CT using One
TOUCH Latch. The integrated Shim System on anterior thermoplastic masks allows
for a minimal invasive approach to height adjustments. Shim adjustments are in
increments of 0.5 mm. Recommended height is 2 mm. The posterior thermoplastic
mask adds support under the patientís neck. The IntegraBite
System is designed to immobilize the intracranial during treatment, allowing
replication of position. Three fiducial markers are placed on the device around
the head of the patient.
CT simulation: Planning CT
was acquired at 1.0 mm slice spacing. After simulation, the DICOM CT, images
were sent to server which was then imported for delineation of target and organ
at risk (OAR). Planning CT was fused with brain MRI. Brain MRI was done in
neutral neck position with no gap, no tilt with sequences at 1 mm interval.
Sequences used included T1, T2 and FLAIR. MRI -CT fusion helped in delineating
OARS and Gross treatment volume (GTV).
Target and OARs delineation: GTV was contoured as gross volume on post-Contrast T1 weighted
MRI sequences. PTV was generated by geometric expansion of GTV + 2 mm margin.
OARs delineated include brainstem, optic nerve, optic chiasm, and lens.
Hippocampal sparing SRS planning was done (7). Fused MRI helped in delineating
the above OARs and contouring.
Radiotherapy technique: Planning
was done on Varian Truebeam equipped with 120 HDMLC
using Eclipse treatment planning system (17.0 - External beam planning). The
calculation algorithm utilized was the Anisotropic Analytical Algorithm
(version 17.0.1). Prescribed doses were 24Gy in 3 fractions. The plan was made
using one co-planar full arc and three non-coplanar partial arcs. An avoidance
sector of 50 degrees was used for the third partial arc (Couch 270). This was
done to avoid exit beam through body via vertex. A non-coplanar beam was used
to reduce skin dose so that beam entry could be done from different angles.
Figures 2 describe the collimator angles.
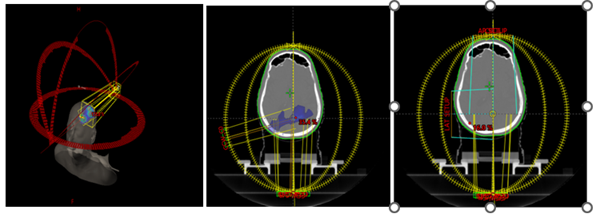
Figure 2a:
Selection of collimator angle; Figure 2b: Spillage for Arc1 at Gantry 255 with
collimator 95 degree (16% dose spill); Figure 2c: Changed collimator angle to
5-degree, to reduce spillage
Plan
optimization was obtained with the help of Optimizer. Photon Optimizer (version
17.0.1) was utilized, which helps to generate shells to reduce dose fall off.
For planning purposes, we have made 2 shells for each PTV. In total, 6 shells
were drawn. First shell for controlling dose falls off at the border of PTV and
the second shell for sharp dose fall outside PTV. Multiple plans (5 plans) were
created.
Plan evaluation: Treatment was
evaluated using plan efficiency indicators derived from DVHs for target
coverage and sparing of OARS. Conformity index (CI), Homogeneity index (HI
RTOG), Quality of coverage were the plan quality metrics used. (8, 9) The dose
falls off outside the target was assessed with Gradient index (GI).Gradient index was less than 2.5 (Table1).
Table 1. Plan efficacy indicators in our case.
|
PTV |
Paddick conformity index (CIPaddick) |
Homogeneity index HI RTOG |
Gradient Index |
|
|
Volume of prescription isodose in the area of interest ie PTV) / PTV volume ◊ Volume of prescription
isodose |
Maximum dose / Prescription dose |
Equivalent radius of 50% isodose Ė Equivalent radius of
prescription isodose. |
|
PTV1 |
0.801 |
1.3 |
2.5 |
|
PTV2 |
0.853 |
1.25 |
|
|
PTV3 |
0.813 |
1.23 |
Quality Assurance (QA): Patient-specific
QA was done with a Pin point 3D chamber using Ruby Phantom. The point dose
verification was done keeping the tolerance as 1 mm.
Challenges, advantages and drawbacks with single isocenter multiple
target stereotactic fractionated radiotherapy
There are
multiple challenges encountered with single isocenter multiple target
stereotactic fractionated radiotherapy. The single isocenter multiple target
stereotactic fractionated radiotherapy involves treating off targets. The
dosimetry and modeling of small MLC opening, which are frequently employed in
multitarget radiosurgery, makes it particularly difficult. Each plan isocenter
is centered on a target when targets are addressed independently, allowing
imaging-based alignment to concentrate mostly on that region of interest.
Rotational errors up to a few degrees usually have a negligible dosimetric effect under these circumstances. On the other
hand, since at least one target must be offset from the point of rotation,
multi-target, single-isocenter SRS treatments are less robust to rotational
errors. The impact of rotational errors on target coverage was examined by
Justin Roper and colleagues in a variety of SRS scenarios (10, 11). The plan
isocenter was placed at the geometric isocenter of three PTVís in our patient.
Some clinics might rely on manual patient repositioning to account for
rotational problems in the initial setup, even though our facility has a
robotic another factor that will affect the dosimetric
impact of rotational errors on the GTV is the margin. After characterizing
rotational uncertainty, target coverage can be predicted using multivariate
regression models with patient specific input characteristics. One benefit of
treating several targets at once is that the treatment period is shortened .Thus, enhanced effectiveness enables the
treatment of more patients.
The biggest
drawback of this technique is its limited availability. Other frequently
discussed drawback is risk of compromised coverage: This issue can be addressed
by placing plan isocenter nearer the smaller PTV to reduce the chance of
compromised coverage. Lastly, not all patients are considered good candidates
for single isocenter multiple target stereotactic fractionated radiotherapy, if
the gap in between the contours is significant, this approach may not be ideal.
Our patient was an ideal candidate due to overlap contours. New optimization
techniques were described by David et al. for VMAT SRS plan of brain tumor
(12). With VMAT SRS more conformal plans can be made in the high and
intermediate dosage regions (about 50% of the Prescription dose), where the
Paddick conformity index (PCI) was enhanced and the dose in the target's core
was noticeably raised while V12 and mean modified gradient index (mGI) were dramatically reduced. These techniques can be
applied to treatment planning for various brain tumors when it is essential to
preserve the surrounding tissue. In protocols 90-05 (13) and 93-05, the
Radiation Therapy Oncology Group (RTOG) proposed the SRS quality assurance and
plan evaluation guidelines based on three parameters: the homogeneity index
(HI), the conformity index (CI) (8) and target coverage.
SRS's
toxicities have been associated with the Paddick conformity index, mGI and V12. Larger the PCI and smaller V12/mGI, the lesser brain toxicity in form of radionecrosis. Side effects profile of SRS include
headache, seizures, localized alopecia, worsening of neurological deficits,
fatigue, radiation dermatitis or radiation-induced brain necrosis as late side
effect (14, 15). Radiation-induced brain necrosis is due to vascular
endothelial damage and demyelination of the white matter (16). Our patient completed
treatment without any major side effects. Planned imaging with the CEMRI brain
shows complete response with no new findings.
Conclusion
Stereotactic radiosurgery is the new standard surgery for multiple
brain metastasis. It doesn't require surgical incisions and is popular and
suitable for patients with primary tumors and brain metastases. Stereotactic
radiosurgery has been effective in brain metastasis. Brain metastasis trials
yield has shown less deterioration of cognition with SRS use, although they do
demonstrate benefits for local control with Combined WBRT therapy. Essential
yet challenging aspect of SRS is dosimetry. It requires a comprehensive Quality
assurance to treatment planning to its delivery. Proton SRS is an uncommon
choice because of its high cost and space requirements. LINAC based VMAT SRS
plans are more conformal with prescribed isodose line upto 75%. Hence
optimization strategies should be applied for better plan outcome.
Author
contribution
†SA and KG write the main script,
revised the script, conceptualized, and prepared figures.
Conflict of
interest
The author
declares no conflict of interest.
Funding
There is no funding.
References
1. Matsuda A, Matsuda T, Shibata A, Katanoda
K, Sobue T, Nishimoto H. (Japan Cancer Surveillance
Research Group). Cancer incidence and incidence rates in Japan in 2008: a study
of 25 population-based cancer registries for the Monitoring of Cancer Incidence
in Japan (MCIJ) project. Jpn J Clin Oncol. 2014;44:388Ė396.
2. Ganz JC. The journey from proton to gamma knife. Prog Brain Res.
2014;215:67-75. 7
3. Suh JH. Stereotactic radiosurgery for the management of brain
metastases. N Engl J Med. 2010 Mar 25;362(12):1119-27.
4. Routman DM, Bian SX, Diao K, et al. The growing importance of
lesion volume as a prognostic factor in patients with multiple brain metastases
treated with stereotactic radiosurgery. Cancer Med. 2018;7:757-764.
5.Yamamoto, Masaaki et al. Stereotactic radiosurgery for patients
with multiple brain metastases (JLGK0901): a multi-institutional prospective
observational study The Lancet Oncology, Volume 15, Issue 4, 387 - 395
6.Chen JC, Girvigian MR. Stereotactic
radiosurgery: instrumentation and theoretical aspects-part 1. Perm J. 2005
Fall;9(4):23-6.
7. Gondi V, Hermann BP, Mehta MP: Hippocampal dosimetry predicts
neurocognitive function impairment after fractionated stereotactic radiotherapy
for benign or low-grade adult brain tumors. Int J Radiat
Oncol Biol Phys. 2012, 15:487-93.
8. Shaw E, Kline R, Gillin M, Souhami L,
Hirschfeld A, Dinapoli R, et al. Radiation Therapy
Oncology Group:Radiosurgery
quality assurance guidelines. Int J Radiat Oncol Biol
Phys. 1993;27:1231Ė9
9. Feuvret L, NoŽl G, Mazeron
JJ, Bey P: Conformity index: a review. Int J Radiat
Oncol Biol Phys. 2006, 1:333-42.
10. Roper J, Chanyavanich V, Betzel G.
Single-Isocenter Multiple-Target Stereotactic Radiosurgery: Risk of Compromised
Coverage. Int J Radiat Oncol Biol Phys. 2015 Nov
1;93(3):540-6.
11. Prentou G, Pappas EP, Logothetis A, Dosimetric impact of rotational errors on the quality of
VMAT-SRS for multiple brain metastases: Comparison between single- and
two-isocenter treatment planning techniques. J Appl Clin Med Phys. 2020
Mar;21(3):32-44.
12. Wang D, DeNittis A, Hu Y. Strategies
to optimize stereotactic radiosurgery plans for brain tumors with
volumetric-modulated arc therapy. J Appl Clin Med Phys. 2020 Mar;21(3):45-51.
13. Shaw E, Scott C, Souhami L, Dinapoli R, Single dose radiosurgical
treatment of recurrent previously irradiated primary brain tumors and brain
metastases: final report of RTOG protocol 90-05. Int J Radiat
Oncol Biol Phys. 2000 May 1;47(2):291-8.
14. Solberg TD, Balter JM, Benedict SH, Fraass
BA, Kavanagh B, Miyamoto C, Pawlicki T, Potters L, Yamada Y. Quality and safety
considerations in stereotactic radiosurgery and stereotactic body radiation
therapy: Executive summary. Pract Radiat
Oncol. 2012 Jan-Mar;2(1):2-9.
15. Redmond KJ, Gui C, Benedict S, et al. Tumor control probability
of radiosurgery and fractionated stereotactic radiosurgery
16. Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic pocess. Radiat Res. 2000
Apr;153(4):357-70.