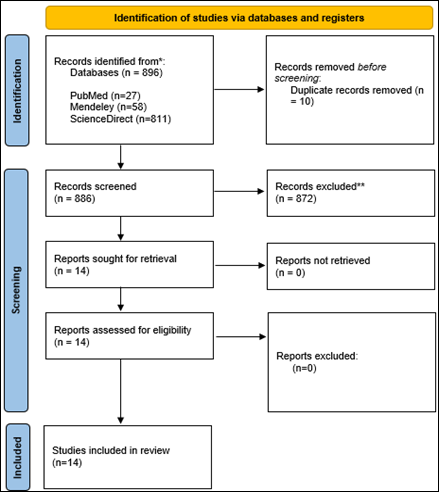
Figure 1. Prisma flow diagram illustrating the study selection process.
The Association
between oral antihypertensive drug use and lung cancer risk in adults: a
systematic review
Shadman
Newaz 1*, Ali Ahmed Shaju 2, Moontasir
Ahmed 1, Ayesha Noor 3, Sarwar Jahan Ratul 1, Marjia
Islam Tisha 1, Samin Sadaf 2, Bohnishikha
Saha 1
1 Tangail
Medical College, Tangail, Bangladesh
2 Dinajpur
Medical College, Dinajpur, Bangladesh
3 Department
of Pharmacy, Jahangirnagar University, Dhaka, Bangladesh
Corresponding Author: Shadman
Newaz
* Email: shadmannewaz11@gmail.com
Abstract
Introduction: Hypertension is a
widespread cardiovascular condition often managed with antihypertensive
medications, including ACE inhibitors (ACEIs), angiotensin receptor blockers
(ARBs), calcium channel blockers (CCBs), and others. Emerging evidence suggests
these medications may influence cancer risk, particularly in lung cancer, a
leading cause of cancer-related mortality worldwide. This systematic review
aims to evaluate the relationship between antihypertensive drugs and lung
cancer risk, focusing on both protective and risk-increasing effects of these
drugs.
Materials and methods: A comprehensive search was conducted across multiple databases
for studies published between January 2015 and February 2025. Eligible studies
included cohort studies, case-control studies, clinical trials, and
observational studies. The review followed PRISMA guidelines for transparency
and comprehensive reporting.
Results: A total of 14 studies—including cohort and case-control
designs—met the inclusion criteria. The findings suggest that ACEIs were
associated with an increased risk of lung cancer, especially with prolonged
use, while ARBs seem to offer protective effects, particularly in certain
populations such as heavy drinkers and males. CCBs, when used in combination
with other antihypertensive drugs, may increase cancer risk, while
α-blockers combined with aspirin show promise in reducing cancer risk, particularly
in older adults. Doxazosin and felodipine have potential in reducing cancer
aggression and improving outcomes through modulation of tumor microenvironments
and immune responses.
Conclusion: The relationship between antihypertensive medications and lung
cancer risk is complex, with ACEIs potentially increasing the risk and ARBs
offering protective effects. Future research should focus on larger prospective
studies, exploring molecular mechanisms and developing personalized treatment
strategies to minimize cancer risk in hypertensive patients. Regular screenings
and careful management of drug interactions are essential for improving
clinical outcomes.
Keywords: Antihypertensive drugs, ACE inhibitors, ARBs, Lung cancer, Calcium
channel blockers, Cancer risk, Systematic review
Hypertension, a
prevalent cardiovascular condition affecting millions globally, is
characterized by persistently elevated blood pressure levels
Lung cancer, one
of the leading causes of cancer-related mortality worldwide, presents a
significant public health challenge
Given the
conflicting evidence and the clinical significance of understanding the
implications of antihypertensive therapy in lung cancer patients, a systematic
review is warranted. This review aims to synthesize the current literature on
the relationship between antihypertensive medications and lung cancer,
evaluating both the potential benefits and risks associated with their use. By
providing a comprehensive overview of existing studies, this review seeks to
inform clinical practice and guide future research directions in this critical
area of oncology and cardiovascular health.
Methods
Study Design and Protocol Registration
This systematic
review was conducted following a predefined protocol that was registered on the
Open Science Framework. The review adhered to the Preferred Reporting Items for
Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure transparency
and comprehensive reporting throughout the review process.
Inclusion and Exclusion Criteria
The review
included studies published between January 2015 and February 2025 that
investigated the relationship between antihypertensive medications and lung
cancer outcomes. Eligible studies were of various designs, including clinical
trials, cohort studies, case-control studies, and observational studies. Only
studies published in English were considered. Studies were included if they
focused on patients diagnosed with hypertension and explored the use of
antihypertensive medications in relation to lung cancer outcomes. Exclusion
criteria included non-English studies, those lacking sufficient data for
extraction, study protocols, and studies addressing other cancer types without
specific reference to lung cancer and hypertension or antihypertensive use. Studies published prior to 2015 were excluded to ensure the inclusion of
more recent and methodologically rigorous research, reflecting advances in
diagnostic techniques, drug classification, and cancer surveillance practices
that have emerged over the past decade.
Search Strategy
A comprehensive
and refined search was conducted across four major electronic databases:
PubMed, ScienceDirect, Cochrane Central Register of Controlled Trials
(CENTRAL), and Mendeley. The search strategy involved a combination of Medical
Subject Headings (MeSH) and free-text terms to
capture studies related to antihypertensive medications and lung cancer
outcomes. The primary concepts of the search were antihypertensive medications,
lung cancer, and hypertension. Specific search terms included:
●
Antihypertensive classes:
"angiotensin-converting enzyme inhibitors" OR "ACE
inhibitors" OR "angiotensin II receptor blockers" OR
"ARBs" OR "beta-blockers" OR "calcium channel
blockers" OR "diuretics" OR "renin-angiotensin system"
OR "antihypertensive agents."
●
Lung cancer terms: "lung cancer" OR
"lung carcinoma" OR "pulmonary carcinoma" OR
"non-small cell lung cancer" OR "small cell lung cancer" OR
"lung neoplasms."
●
Lung cancer subtypes:
"adenocarcinoma" OR "squamous cell carcinoma" OR
"small cell lung cancer" OR "non-small cell lung cancer."
Additionally,
keywords such as "lung cancer incidence," "lung cancer
progression," "lung cancer recurrence," "lung cancer
mortality," and "lung cancer survival" were combined with terms
related to antihypertensives. To capture a broader range of relevant studies,
terms were also expanded to include related side effects, mechanisms, and risk
assessments, such as:
●
"hypertension
treatment" OR "cardiovascular drugs" AND "lung cancer
risk."
●
"antihypertensive
side effects" AND "lung cancer survival."
●
"risk of lung
cancer" AND "antihypertensive drugs."
A second search
iteration targeted grey literature sources by searching databases like Web of
Science, Scopus, and Google Scholar. Reference lists of key studies and reviews
were also screened to ensure no relevant studies were overlooked. The search
covered studies published from January 2015 to February 2025, and the database
searches were initially performed on January 26, 2025, with an update conducted
on February 26, 2025.
Screening and Data Extraction
The screening
process was managed using Rayyan software, which allowed for the removal of
duplicates and facilitated the title and abstract screening. Two independent
reviewers (MA and SR) conducted the initial screening, with disagreements
resolved by a third reviewer (MT). Full-text reviews were conducted for studies
that met the inclusion criteria.
Data extraction
was performed using a predesigned Excel spreadsheet that captured key details,
including study design, patient population, type of antihypertensive
medications used, lung cancer outcomes, and major findings. Data extraction was
carried out by SN, with 50% of the data verified independently by AS and SS to
ensure accuracy.
Quality Appraisal
Although the
primary aim of this systematic review was to summarize and map the existing
evidence rather than to critically appraise study quality, a descriptive
evaluation of study limitations and potential biases was performed for each
study. Formal quality appraisal tools, such as the Newcastle-Ottawa Scale (for
cohort and case-control studies), were applied where appropriate, but no
studies were excluded based on quality criteria.
Data Synthesis
Due to the
heterogeneity in study designs and outcomes, a narrative synthesis was
conducted. A meta-analysis (quantitative pooling of data) was not performed due
to variations in study methods, populations, and outcome measures across the
included studies. Heterogeneity was assessed qualitatively by comparing study
designs, patient characteristics, interventions, and outcome definitions across
studies.
For the purpose
of this review, "lung cancer outcomes" encompassed a range of
endpoints, including cancer incidence, disease progression, treatment response,
survival rates (overall and disease-specific), and mortality.
The results were
synthesized to provide a broad overview of the available evidence on the
relationship between antihypertensive medications and lung cancer outcomes.
Assessment of Bias
Bias assessment
was carried out using established tools and guidelines to ensure a rigorous
evaluation process. The ROBINS-I tool was employed to assess the quality and
risk of bias in the included studies. This evaluation considered various
factors, such as selection bias, performance bias, detection bias, and
reporting bias. Multiple researchers independently reviewed each study to
maintain consistency and objectivity in the assessment.
Although no
studies were excluded based on bias ratings, findings from studies assessed as
having a high risk of bias were interpreted with caution during synthesis.
Where applicable, subgrouping and narrative comparisons were used to explore
differences in outcomes based on risk of bias. No formal sensitivity analysis
was performed; however, the level of bias was qualitatively considered when
drawing final conclusions about the strength and reliability of the evidence.
This
methodological approach aimed to provide a comprehensive understanding of
potential biases influencing study outcomes and to enhance the reliability of
the systematic review’s findings.
Results
The review
process details are depicted in the PRISMA flowchart (Figure 1). A total of 896
records were identified through database searches, including Science Direct
(n=811), PubMed (n=27), and Mendeley (n=58).
After removing 10 duplicate records, 886 records remained for
title and abstract screening. Following this initial screening, 872 records
were excluded based on irrelevance to the study objectives. Subsequently, 14
full-text articles were assessed for eligibility. Ultimately, all 14 studies
were included in the systematic review for further in-depth analysis of the
core relationship between antidiabetic drugs and the risk of developing liver
cancer.

Figure 1. Prisma flow diagram illustrating the study selection process.
Below table
(Table 1) summarizes the geographical distributions of the included studies.
Table 1. Country distribution of included studies.
|
Country |
Count |
|
Korea |
2 |
|
Hong Kong |
2 |
|
East Asia |
1 |
|
Shanghai |
1 |
|
United Kingdom |
1 |
|
Unidentified |
7 |
Seven studies
lack country identification, while five studies are from Korea, Hong Kong, East
Asia, Shanghai, and the United Kingdom. The lack of geographical information in
these seven studies limits the ability to generalize the findings across
different populations and healthcare systems. Further details on the study
locations would improve the applicability of the results. The studies included
in this review employed a variety of designs (Table 2), with the majority being
cohort studies. Additionally, there were case-control studies, a Mendelian
randomization study, a case report, a pre-clinical model, and an in-vitro
model. This diverse range of study designs provides valuable insights, though
the observational nature of many studies may introduce potential biases.
Among the
included studies, one case report, one pre-clinical model, and one in vitro
study were identified alongside larger epidemiological studies. These studies
were synthesized separately from cohort and case-control studies to preserve
interpretive clarity and account for methodological heterogeneity. While they
did not contribute directly to population-level outcome trends, they provided
valuable mechanistic insights into potential biological pathways through which
antihypertensive medications may influence lung cancer development or
progression. Their findings were used to support or contextualize associations
observed in clinical studies but were interpreted cautiously due to inherent
limitations in generalizability.
Table 2. Methodological Designs of Included Studies.
|
Study design |
Count |
|
Cohort |
9 |
|
Case-control |
2 |
|
Mendelian randomization study |
1 |
|
Systematic review |
1 |
|
Case report |
1 |
|
Pre-clinical Model |
1 |
|
In-vitro Model |
1 |
Key Characteristics of the Included Studies
The table (Table
3) below summarizes the key characteristics of the studies included in this
review, including country, study design, total participants, age, gender, and
limitations. This provides an overview of the diversity in study contexts and
methodologies. While most included studies reported comprehensive data such as
country of origin and participant demographics, a few lacked such details.
These studies were retained to ensure inclusivity of all relevant evidence on
the relationship between antihypertensive medications and lung cancer outcomes.
Despite the missing contextual information, these studies provided valuable
outcome data and mechanistic insights that contributed meaningfully to the
overall synthesis. Their inclusion was justified by their relevance to the
review question and methodological adequacy in other areas, as assessed through
the bias appraisal process. To minimize potential impact, findings from these
studies were interpreted with appropriate caution in the narrative synthesis.
Table 3 Key characteristics of studies included in the systematic review.
|
References |
Country |
Design |
Total Participants |
Age |
Gender |
Limitations |
|
(27) |
East Asia |
Cohort |
228 |
N/A |
Both |
Non-randomized, retrospective design with
small sample size, potential biases, and lack of molecular exploration. |
|
(28) |
N/A |
In-vitro |
N/A |
N/A |
N/A |
In vitro model limits accuracy, no in vivo
confirmation, lacks consideration of drug interactions or adverse effects. |
|
(29) |
N/A |
Pre-clinical model |
N/A |
N/A |
N/A |
Small sample size, reliance on preclinical
models, and insufficient mechanistic evidence. |
|
(30) |
Korea |
Cohort |
0.3 million |
≥40 years |
Both |
Residual confounding, retrospective design,
and limited generalizability due to a specific population. |
|
(31) |
N/A |
Mendelian randomization study |
N/A |
N/A |
N/A |
Long-term genetic impact focus, limited
generalizability, and lack of RCTs weakening causal conclusions. |
|
(32) |
N/A |
Case-control |
178 |
≥18 |
Both |
Small sample size, retrospective design, and
unaddressed comorbidities/genetic factors. |
|
(33) |
Hong Kong |
Cohort |
6,592 anti-hypertensive users, 84,116
non-users |
N/A |
Both |
Lack of smoking status consideration,
unmeasured confounders, and potential inapplicability to the Hong Kong
population. |
|
(34) |
Shanghai |
Cohort |
4,970 cancer cases |
N/A |
Both |
Missing dosage information, pharmacological
classes, and limited applicability outside Shanghai. |
|
(35) |
Worldwide |
Systemic review |
N/A |
N/A |
N/A |
Potential biases (selection, recall), and
inconsistent prior research affecting causality and generalizability. |
|
(36) |
Hong Kong |
Cohort |
6,592 and 84,116 lung cancer cases |
N/A |
N/A |
Lack of causality data and missing data on
rare tumors and medication details. |
|
(37) |
N/A |
Case-control |
4,174 lung cancer cases |
N/A |
N/A |
N/A |
|
(38) |
United Kingdom |
Cohort |
992,061 |
N/A |
N/A |
Missing smoking data, unmeasured
confounders, and potential misclassification of results. |
|
(39) |
N/A |
Case report |
1 |
N/A |
Female |
Unaccounted genetic/environmental factors
and no long-term data on cancer outcomes. |
|
(40) |
South Korea |
Cohort |
60,469 subjects |
N/A |
Both |
Observational design with potential
misclassification and missing subtype-specific data. |
Risk of Bias Assessment
The risk of bias
across the included non-randomized studies was evaluated using the ROBINS-I
tool. Overall, most studies demonstrated concerns regarding bias risk in
multiple domains. The most frequent issues were related to the selection of
participants, deviations from intended interventions, and
reporting of outcomes, where many studies lacked sufficient information
or exhibited methodological concerns. A few studies were judged to have a high
risk of bias, mainly due to confounding and missing outcome data. Only a
limited number of studies were rated as low risk across all domains. The
domain-wise distribution of the risk of bias is visually summarized in the
figures (Figure 2 A and B) below. Figure 2 presents a comprehensive summary of
the risk of bias assessment across included studies. Figure 2 A provides a
visual overview of the risk of bias for each individual study, categorized into
five domains. Each domain is evaluated using a traffic light color-coding
system—green (+) for low risk, yellow (–) for some concerns, and red (×) for
high risk. Most studies exhibit “some concerns” across multiple domains, with a
smaller number rated as “low risk” throughout. High risk assessments are
predominantly observed in D2, indicating deviations from intended
interventions.
A
Figure 2B
illustrates the distribution of risk of bias judgments across all studies by
domain, represented as a bar chart. The highest proportion of “some concerns”
is seen consistently across most domains. Domains D1 and D2 show the greatest
frequency of “high risk” assessments, while domain D3 (missing outcome data)
has the fewest concerns, with a relatively higher proportion rated as “low
risk.” Overall, only a limited number of studies are free from bias across all
domains.
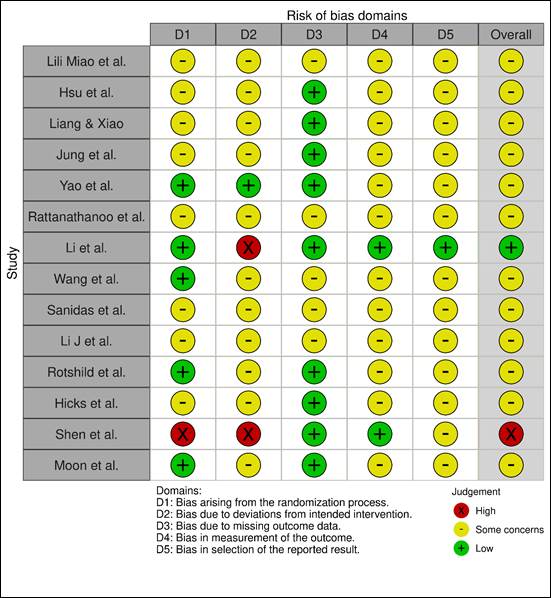
Figure 2. Summary and domain-level distribution of risk of bias in included
non-randomized studies
Legend (Applicable to Both Figures):Green (+):
Low risk of bias. Yellow (–): Some concerns regarding bias. Red (×): High risk
of bias. D1: Bias arising from the randomization process. D2: Bias due to
deviations from intended interventions. D3: Bias due to missing outcome data. D4:
Bias in measurement of the outcome. D5: Bias in selection of the reported
result. Overall: Combined assessment of all domains for each study.
Main Findings of the Studies based on
Antihypertensive Drugs Classification
Key findings of
the included studies are given below (Table 4) according to the different
classes of anti-diabetic drugs.
Table 4. Main findings of the studies based on antihypertensive drugs
classification.
|
Drug Class |
Specific Findings |
References |
|
ACE Inhibitors (ACEIs) |
ACEIs linked to increased lung cancer risk,
especially with prolonged use. Risk observed in non-smokers and higher after
five years. |
(31,38) |
|
ARBs |
ARBs reduced lung cancer risk compared to
ACEIs, particularly in men and heavy drinkers, with stronger effects after
prolonged use. |
(30,40) |
|
Calcium Channel Blockers (CCBs) |
CCBs associated with a slightly higher risk
of lung cancer, especially thyroid and lung cancers, when multiple
antihypertensive drugs are used. |
(33,34,37) |
|
α-blockers |
α-blockers, especially when combined
with aspirin, reduced lung cancer risk, with the greatest benefit seen in
older adults. |
(33,36) |
|
Doxazosin |
Doxazosin reduced cancer cell aggression
and metastasis in NSCLC models, showing promise as a therapeutic option. |
(28) |
|
Felodipine |
Felodipine slowed tumor
growth and improved outcomes when combined with immune therapies, possibly by
influencing NFAT1. |
(29) |
●
ACEIs are associated with an
increased risk of lung cancer, particularly with prolonged use
●
ARBs offer a protective effect,
reducing lung cancer risk compared to ACEIs, particularly in certain
populations
●
CCBs may increase cancer risk,
especially with multi-drug antihypertensive combinations
●
α-blockers and aspirin combination enhance cancer prevention, especially in older
adults
●
Doxazosin and Felodipine have promising potential in reducing cancer cell
aggression and improving outcomes
Table below
(Table 5) includes influencing factors for lung cancer due to hypertension
management. The influencing factors identified in the studies include the
impact of ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) on
survival outcomes in NSCLC patients
Table 5. Influencing factors for lung cancer due to
hypertension management according to the included studies.
|
References |
Influencing factor |
|
(27) |
Use of ACE inhibitors (ACEIs) and
angiotensin receptor blockers (ARBs) in NSCLC patients to evaluate their
effect on survival outcomes. |
|
(28) |
Vasculogenic mimicry (VM) in NSCLC is
influenced by VEGF-A, VE-cadherin, EphA2/AKT/mTOR/MMP pathway, and
epithelial-mesenchymal transition (EMT) markers (vimentin, fibronectin).
Doxazosin inhibits VM by targeting these pathways. |
|
(29) |
Felodipine's effectiveness in lung squamous
cell carcinoma (LUSC) is impacted by NFAT1 expression, immune system
modulation, tumor resistance to immune checkpoint
blockers (ICBs), and its role in treating both hypertension and cancer. |
|
(30) |
Prolonged ARB use significantly lowers the
risk of lung, hepatic, and gastric cancers, especially in males and heavy
drinkers, by inhibiting AT1 receptors while preserving AT2 receptor benefits.
Effects vary by cancer type and demographics. |
|
(31) |
Genetic variants (e.g., rs118121655,
rs360206) and ACEI-induced cough increase lung cancer risk, particularly in
Europeans with small-cell lung cancer (SCLC) and adenocarcinoma. Bradykinin
and protachykinin-1 accumulation may contribute to this risk. |
|
(32) |
Dyslipidemia and family
history of lung cancer significantly elevate cancer risk in hypertensive
patients, while calcium channel blockers (CCBs) show no association. Smoking
and comorbidities were not linked to lung cancer. |
|
(33) |
Long-term use of ACEIs, ARBs, and
α-blockers reduces lung cancer risk, with greater benefits observed in
older adults (above 65) and males. Aspirin combined with α-blockers
further lowers risk, but smoking status was not considered in the analysis. |
|
(34) |
Increased cancer risk is linked to the use
of multiple antihypertensive drug classes, with hazard ratios of 1.22 for
two-drug combinations and 1.22 for three or more classes. |
|
(35) |
Cancer risk is associated with ACEIs, ARBs,
CCBs, beta-blockers (BBs), and diuretics. Research also examines rare side
effects and potential links between antihypertensive drugs and malignancy. |
|
(36) |
Cancer risk varies based on drug mechanisms
(RAS inhibitors may protect, while CCBs and thiazides might increase risk),
treatment duration, dosage, and patient-specific factors such as
comorbidities, lifestyle, and genetics. |
|
(37) |
Prolonged ACEI use (over five years) is
associated with an increased lung cancer risk, peaking at 10 years. The
accumulation of bradykinin and substance P in the lungs may contribute to tumor growth. Risk persists in non-smokers and is higher
than with ARBs. |
|
(38) |
Drug-drug interactions (CYP3A4-mediated
competition between gefitinib, nifedipine, and simvastatin), genetic factors
(wild-type CYP3A4, weak CYP2D6 metabolism), and patient non-compliance impact
drug toxicity and treatment outcomes. |
|
(39) |
ARB use lowers lung cancer risk more than
CCBs, particularly in women, never-smokers, non-drinkers, and those without
COPD. Lifestyle factors (smoking, alcohol) and comorbidities (diabetes, dyslipidemia) influence treatment effects. |
Discussion
The relationship between antihypertensive
medications and cancer risk has been explored across various studies with mixed
results
Recommendations of the Studies
Recommendations from the included studies
with their key insights are given in the table below (Table 6). The recommendations across
the studies emphasize the need for more extensive research and careful patient
management. Larger prospective trials should be conducted to confirm findings,
reduce bias, and explore the biological mechanisms behind observed effects
Clinical Implications of the Study
This study
highlights the importance of considering the long-term effects of
antihypertensive drugs on cancer risk, particularly in lung cancer. ACE
inhibitors (ACEIs) may increase the risk, while angiotensin receptor blockers
(ARBs) show protective effects. The findings suggest that clinicians should
evaluate the type of antihypertensive prescribed based on patient demographics,
comorbidities, and potential cancer risks. Additionally, drugs like doxazosin
and felodipine, which show promise in reducing cancer aggression and improving
immune responses, may offer new therapeutic options.
Limitations of the Study
The study's
limitations include reliance on observational designs, small sample sizes in
some studies, lack of long-term follow-up data, and potential biases such as
confounding factors (e.g., smoking, alcohol use, genetic variations).
Additionally, the studies did not fully address the molecular mechanisms of
antihypertensives' effects on cancer development. The generalizability of
findings is also limited due to specific population settings and regional
differences.
Table 6. Key recommendations of selected studies.
|
References |
Recommendations |
Key Insights |
|
(27) |
Conduct larger, prospective randomized trials to validate findings and
reduce bias. Explore biological mechanisms and ensure diverse patient
populations to improve generalizability. |
Larger trials and exploration of biological mechanisms are necessary to
confirm the results and improve clinical applicability. |
|
(28) |
Conduct further preclinical and clinical trials to confirm doxazosin's
anti-vasculogenic mimicry effects and its potential as an add-on therapy for
NSCLC. |
Doxazosin should be investigated further, particularly for its
potential in combination therapies targeting cancer metastasis. |
|
|
Repurpose felodipine for cancer therapy and assess its combination with
immune checkpoint blockers (ICBs). Explore NFAT1 as a target for treatment. |
Felodipine shows promise for enhancing immune responses in cancer
therapy, and its use in combination therapies warrants further investigation. |
|
(30) |
Consider ARBs as a safer option for long-term use to reduce cancer
risks in hypertensive patients, particularly in men and heavy drinkers. |
ARBs offer protective effects against certain cancers, especially in
specific demographic groups like men and those who consume alcohol. |
|
(31) |
Monitor genetic variants (e.g., rs360206) in ACEI users and consider
ARBs for those who experience ACEI-induced cough. |
Genetic monitoring could help reduce lung cancer risk in patients
taking ACEIs, while ARBs should be considered as an alternative. |
|
(32) |
Continue using CCBs for hypertension management, as they do not
increase lung cancer risk. Focus on managing dyslipidemia
and family history of lung cancer. |
Managing other risk factors like dyslipidemia
and family history is critical, as CCBs do not contribute to increased lung
cancer risk. |
|
(33) |
Explore the combination of antihypertensives (ACEIs, ARBs,
α-blockers) with aspirin for lung cancer prevention, especially in older
adults and males. |
The combination of antihypertensives with aspirin could be beneficial
for lung cancer prevention, particularly in older adults and males. |
|
(34) |
Conduct randomized controlled trials to confirm causality and explore
the impact of different drug combinations on cancer risk. |
More large-scale RCTs are needed to clarify the link between
antihypertensives and cancer risk, with a focus on drug combinations. |
|
(35) |
Monitor long-term use of certain antihypertensive drugs like CCBs and
thiazides, and perform regular cancer screenings for high-risk patients. |
Regular monitoring and cancer screening are essential for hypertensive
patients, particularly those on certain antihypertensive medications. |
|
(36) |
Replicate findings on ACEI-related lung cancer risks in other settings
and monitor long-term use. Explore newer drugs like sacubitril/valsartan. |
Long-term monitoring of ACEI users is crucial, with a focus on
alternative treatments like ARBs and newer medications such as
sacubitril/valsartan. |
|
(37) |
Investigate the risk increase of lung cancer with prolonged CCB use,
and consider patient-specific factors in treatment. |
Prolonged use of CCBs may increase lung cancer risk, making
individualized treatment decisions essential. |
|
(38) |
Consider alternative treatments to ACEIs, particularly for patients
with long-term use, and explore newer antihypertensive drugs. |
Prolonged use of ACEIs was associated with increased lung cancer risk;
ARBs and newer drugs like sacubitril/valsartan could offer safer
alternatives. |
|
(39) |
Avoid CYP3A4-mediated drug interactions in cancer patients and foster
multidisciplinary collaboration for managing drug toxicity. |
Drug-drug interactions should be avoided by choosing alternative
medications like valsartan and rosuvastatin, and collaboration between
healthcare providers is essential. |
|
(40) |
Consider ARBs for hypertensive patients at high risk of lung cancer,
particularly non-smokers, women, and non-drinkers. |
ARBs are recommended for hypertensive patients at higher risk for lung
cancer, especially those with specific lifestyle factors. |
Author contribution
SN developed the methodology and wrote the methodology section. SN
also conducted data extraction using a predesigned Excel spreadsheet, capturing
key study details, including study design, patient population, type of
antihypertensive medications used, lung cancer outcomes, and major findings.
Additionally, SN oversaw the entire review process and coordinated the
writing of the manuscript. AS independently verified 50% of the
extracted data to ensure accuracy and consistency. MA also wrote the results
section, contributed to the final review of the manuscript, played a role in
developing the study design, and assisted in refining the methodology section. MA
contributed to refining the search strategy, participated in the full-text
review process, and assisted in synthesizing the extracted data. MA also
built the tables and diagrams for the manuscript and helped review the
methodology section. AN independently conducted the title and abstract
screening using Rayyan software, ensuring the initial selection of studies. AN
also conducted the full-text review for studies meeting the inclusion criteria
and wrote the discussion section. SR independently verified 50% of the
extracted data alongside MA to enhance data accuracy. SR also
contributed to refining the study methodology and participated in manuscript
revisions. MT wrote the introduction section and assisted in optimizing
the search strategy. MT also played a role in screening full-text
articles and contributed to drafting and reviewing the discussion section. SS
independently conducted the title and abstract screening using Rayyan software,
ensuring the initial selection of studies. SS also wrote the conclusion
section and participated in discussions regarding study inclusion and exclusion
criteria. BS contributed to writing the discussion section and provided
critical revisions to improve clarity and coherence. BS also
participated in reviewing the final manuscript to ensure consistency and
accuracy. NT played a role in the quality assessment of included studies
and assisted in synthesizing the extracted data. NT also contributed to
reviewing the discussion and conclusion sections to ensure alignment with the
study objectives. All authors contributed to the conception and design of the
study, provided input on data interpretation, and participated in manuscript
revisions. All authors approved the final version before submission.
Funding
There
is no funding.
Conflicts
of interest
There
are no conflicts of interest.
References
1. Mills KT, Stefanescu A, He J. The global
epidemiology of hypertension. Nat Rev Nephrol. 2020 Apr 1;16(4):223.
2. Chaturvedi A, Zhu A, Gadela
NV, Prabhakaran D, Jafar TH. Social Determinants of Health and Disparities in
Hypertension and Cardiovascular Diseases. Hypertension. 2024 Mar 1;81(3):387–99.
3. Kifle ZD, Adugna M, Chanie GS, Mohammed A.
Prevalence and associated factors of hypertension complications among
hypertensive patients at University of Gondar Comprehensive Specialized
Referral Hospital. Clin Epidemiol Glob Health. 2022
Jan 1;13:100951.
4. Seravalle G,
Grassi G. Essential Hypertension. Primer on the Autonomic Nervous System,
Fourth Edition. 2023 Jul 20;467–70.
5. Oster JR, Materson
BJ, Perez-Stable E. Antihypertensive Medications. South Med J. 2023 May 8;77(5):621–30.
6. Jones KE, Hayden SL, Meyer HR, Sandoz JL,
Arata WH, Dufrene K, et al. The Evolving Role of Calcium Channel Blockers in
Hypertension Management: Pharmacological and Clinical Considerations. Current
Issues in Molecular Biology 2024, Vol 46, Pages 6315-6327. 2024 Jun 22;46(7):6315–27.
7. Sever PS, Messerli FH. Hypertension
management 2011: optimal combination therapy. Eur
Heart J. 2011 Oct 1;32(20):2499–506.
8. Elendu C,
Amaechi DC, Elendu TC, Amaechi EC, Elendu ID. Dependable approaches to hypertension
management: A review. Medicine. 2024 Jun 14;103(24):e38560.
9. Felkle D, Jarczyński M, Kaleta K, Zięba K, Nazimek K. The
immunomodulatory effects of antihypertensive therapy: A review. Biomedicine
& Pharmacotherapy. 2022 Sep 1;153:113287.
10. Elendu C, Amaechi
DC, Elendu TC, Amaechi EC, Elendu
ID. Dependable approaches to hypertension management: A review. Medicine
(United States). 2024 Jun 14;103(24):e38560.
11. Schwalm JD, Joseph P, Leong D, Lopez-Lopez
JP, Onuma O, Bhatt P, et al. Cardiovascular disease
in the Americas: optimizing primary and secondary prevention of cardiovascular
disease series: cardiovascular disease in the Americas. Lancet Regional Health
- Americas. 2025 Feb 1;42:100964.
12. Felkle D, Jarczyński M, Kaleta K, Zięba K, Nazimek K. The
immunomodulatory effects of antihypertensive therapy: A review. Biomedicine
& Pharmacotherapy. 2022 Sep 1;153:113287.
13. Stewart J, Addy K, Campbell S, Wilkinson P.
Primary prevention of cardiovascular disease: Updated review of contemporary
guidance and literature. JRSM Cardiovasc Dis. 2020 Jan;9:2048004020949326.
14. Zhou J, Xu Y, Liu J, Feng L, Yu J, Chen D.
Global burden of lung cancer in 2022 and projections to 2050: Incidence and
mortality estimates from GLOBOCAN. Cancer Epidemiol. 2024 Dec 1;93:102693.
15. Didkowska J,
Wojciechowska U, Mańczuk M, Lobaszewski
J. Lung cancer epidemiology: contemporary and future challenges worldwide. Ann
Transl Med. 2016 Apr 1;4(8):150–150.
16. Bray F, Laversanne
M, Sung H, Ferlay J, Siegel RL, Soerjomataram
I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and
mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024
May 1;74(3):229–63.
17. Thandra KC, Barsouk A, Saginala K, Aluru JS, Barsouk
A. Epidemiology of lung cancer. Contemp Oncol. 2021;25(1):45.
18. Zhou J, Xu Y, Liu J, Feng L, Yu J, Chen D.
Global burden of lung cancer in 2022 and projections to 2050: Incidence and
mortality estimates from GLOBOCAN. Cancer Epidemiol. 2024 Dec 1;93:102693.
19. Li C, Lei S, Ding L, Xu Y, Wu X, Wang H, et
al. Global burden and trends of lung cancer incidence and mortality. Chin Med
J (Engl). 2023 Jul 5;136(13):1583.
20. Totolici S, Vrabie
AM, Badila E, Weiss E. Onco-Hypertension: A Continuously Developing Field
between Cancer and Hypertension. Int J Mol Sci . 2024
Mar 1;25(6):3442.
21. Mao X, Wu S, Huang D, Li C. Complications
and comorbidities associated with antineoplastic chemotherapy: Rethinking drug
design and delivery for anticancer therapy. Acta Pharm Sin B. 2024 Jul 1;14(7):2901–26.
22. Yang R, Zhang Y, Liao X, Yao Y, Huang C, Liu
L. The Relationship Between Anti-Hypertensive Drugs and Cancer: Anxiety to be
Resolved in Urgent. Front Pharmacol. 2020 Dec 14;11:610157.
23. Yang R, Zhang Y, Liao X, Yao Y, Huang C, Liu
L. The Relationship Between Anti-Hypertensive Drugs and Cancer: Anxiety to be
Resolved in Urgent. Front Pharmacol. 2020 Dec 14;11:610157.
24. Everatt R, Kuzmickienė
I, Brasiūnienė B, Vincerževskienė
I, Intaitė B, Cicėnas
S, et al. Postdiagnostic use of antihypertensive
medications and survival in colorectal, lung, corpus uteri, melanoma and
kidney cancer patients with hypertension. BMC Cancer. 2025 Dec 1;25(1):38.
25. Catarata MJ,
Ribeiro R, Oliveira MJ, Cordeiro CR, Medeiros R. Renin-Angiotensin System in
Lung Tumor and Microenvironment Interactions.
Cancers (Basel). 2020 Jun 1;12(6):1457.
26. Li J, Lam ASM, Yau STY, Yiu KKL, Tsoi KKF.
Antihypertensive treatments and risks of lung Cancer: a large population-based
cohort study in Hong Kong. BMC Cancer. 2021 Dec 1;21(1):1202.
27. Miao L, Chen W, Zhou L, Wan H, Gao B, Feng
Y. Impact of Angiotensin I-converting Enzyme Inhibitors and Angiotensin II
Type-1 Receptor Blockers on Survival of Patients with NSCLC. Sci Rep. 2016 Feb
17;6.
28. Hsu JL, Leu WJ, Hsu LC, Hsieh CH, Guh JH.
Doxazosin inhibits vasculogenic mimicry in human non‑small cell lung
cancer through inhibition of the VEGF‑A/VE‑cadherin/mTOR/MMP
pathway. Oncol Lett . 2024 Apr 1;27(4).
29. Liang SY, Xiao HK. The antihypertensive
felodipine shows synergistic activity with immune checkpoint blockade and
inhibits tumor growth via NFAT1 in LUSC. Open Med
(Wars). 2023 Jan 1;18(1).
30. Jung MH, Lee JH, Lee CJ, Shin JH, Kang SH,
Kwon CH, et al. Effect of angiotensin receptor blockers on the development of
cancer: A nationwide cohort study in korea. J Clin Hypertens (Greenwich). 2021 Apr 1;23(4):879–87.
31. Yao T, Wu Z, Wang Z, Chen L, Liu B, Lu M, et
al. Association between angiotensin-converting enzyme inhibitor-induced cough
and the risk of lung cancer: a Mendelian randomization study. Front Pharmacol. 2023;14.
32. Rattanathanoo R, Chindaprasirt J, Boonsawat W, Limpawattana P, Khamsai S, Sawanyawisuth K. Are calcium channel blockers related to
lung cancer? Drug Target Insights. 2023 Jan 18;17:54–7.
33. Li J, Lam ASM, Yau STY, Yiu KKL, Tsoi KKF.
Antihypertensive treatments and risks of lung Cancer: a large population-based
cohort study in Hong Kong. BMC Cancer. 2021 Dec 11;21(1):1202.
34. Wang S, Xie L, Zhuang J, Qian Y, Zhang G,
Quan X, et al. Association between use of antihypertensive drugs and the risk
of cancer: a population-based cohort study in Shanghai. BMC Cancer. 2023 Dec 1;23(1).
35. Sanidas E, Velliou M, Papadopoulos D, Fotsali
A, Iliopoulos D, Mantzourani M, et al.
Antihypertensive Drugs and Risk of Cancer: Between Scylla and Charybdis. Am J Hypertens. 2020 Dec 1;33(12):1049–58.
36. Li J, Lam ASM, Yau STY, Yiu KKL, Tsoi KKF.
Antihypertensive treatments and risks of lung Cancer: a large population-based
cohort study in Hong Kong. BMC Cancer. 2021 Dec 1;21(1).
37. Rotshild V,
Azoulay L, Feldhamer I, Perlman A, Glazer M, Muszkat M, et al. Calcium Channel Blockers and the Risk
for Lung Cancer: A Population-Based Nested Case-Control Study. Ann Pharmacother .
2019 May 1;53(5):445–52.
38. Hicks BM, Filion KB, Yin H, Sakr L, Udell
JA, Azoulay L. Angiotensin converting enzyme inhibitors and risk of lung
cancer: population based cohort study. BMJ. 2018;363.
39. Shen X, Fan G, Liu G, Wang F, Li Q, Liu X,
et al. Severe adverse cutaneous reactions induced by gefitinib combined with
antihypertensive and antihyperlipidemic drugs in lung cancer: a case report.
Anticancer Drugs. 2022 Jan 1;33(1):E802–7.
40. Moon S, Lee HY, Jang J, Park SK. Association
Between Angiotensin II Receptor Blockers and the Risk of Lung Cancer Among
Patients With Hypertension From
the Korean National Health Insurance Service-National Health Screening Cohort.
J Prev Med Public Health. 2020 Nov 1;53(6):476–86.
41. Ni H, Rui Q, Zhu X, Yu Z, Gao R, Liu H, et
al. Antihypertensive drug use and breast cancer risk: a meta-analysis of
observational studies. Oncotarget. 2017 Jul 10;8(37):62545–60.
42. Li CI, Malone KE, Weiss NS, Boudreau DM,
Cushing-Haugen KL, Daling JR. Relation between use of antihypertensive
medications and risk of breast carcinoma among women ages 65–79 years. Cancer.
2003 Oct 1;98(7):1504–13.
43. Copland E, Canoy D, Nazarzadeh
M, Bidel Z, Ramakrishnan R, Woodward M, et al.
Antihypertensive treatment and risk of cancer: an individual participant data
meta-analysis. Lancet Oncol . 2021 Apr 1; 22(4):558–70.