Long term clinical outcomes in patients with rectal
adenocarcinoma treated at a Tertiary Care Centre in South India
Amirthvarshan A 1,
Lijeesh A L 1, Roshni S 1*,
Mulla P A 2, Sivanandan C D 1,
Sajeed A 1, Arun Sankar 1, Geethi M H 1, Aleyamma Mathew 3
1 Department of Radiation Oncology, Regional Cancer Centre,
Thiruvananthapuram, India
2 Department of Radiation Oncology, Mangalore Institute of Oncology, Mangaluru, Karnataka, India
3 Division of Cancer epidemiology and Biostatistics, Regional cancer
Centre, Thiruvananthapuram, India
* Corresponding Author: Roshni
S
* Email: roshnisyampramod@gmail.com
Abstract
Introduction: Treatment of rectal adenocarcinoma involves multi-disciplinary approach
which includes radiation, surgery and chemotherapy. Our study aims to assess
the clinical outcomes of patients with rectal adenocarcinoma treated in a
tertiary care centre in South India.
Materials and methods: A retrospective content-based analysis was made of 131 patients
diagnosed and treated for adenocarcinoma rectum in a tertiary care hospital
during the period of 1st January 2014 to 31st December 2015. The primary
objectives were to assess disease free survival (DFS) and overall survival
(OS).
Results: Of the 131 patients, 82 were males and 49 were females with a median
age of 59 years. Stage II and Stage III disease together contributed to 65.6%
of study population. After a median follow up of 105.8 months, the 8-year
overall OS and DFS were found to be 77.2% and 78.8% respectively. In the
univariate analysis, stage of the disease and histology were found to be
significant factors in determining the overall survival (p<0.05).
Multivariate analysis showed poorly differentiated adenocarcinoma emerged as
significant factor affecting overall survival. About 31 (23.6%) patients showed
disease recurrence. Of the 31 patients, pelvic recurrence occurred in 26%
patients and among the distant metastases, liver was the most common site (33%)
followed by lung (22%).
Conclusion: The disease free survival and overall
survival of our patients were comparable with the available published data.
Keywords: Rectal cancer, Histology, Young adults, Survival rates, Prognosis
Introduction
Rectal cancer had been primarily considered a disease of the elderly,
which mostly occur after the fifth decade of life but younger people aged less
than 50 years diagnosed with colorectal cancer have been on the rise recently
(3). Most of the patients present with locally advanced disease having a 5-year
survival of 73% as per National Cancer Institute's (NCI's) Surveillance,
Epidemiology, and End Results (SEER) program and the Centers
for Disease Control and Prevention's (CDC's) National Program of Cancer
Registries (4). But the 5-year survival rate of locally advanced rectal cancer
in India is 40% which is one of the lowest in the world (5).
The standard
treatment in early-stage disease is primary surgery with or without adjuvant
treatment. In locally advanced disease, the standard of care is neoadjuvant
treatment followed by surgery with or without adjuvant chemotherapy. Assessment
of survival is the strongest parameter in oncologic outcomes. The complexity of multidisciplinary
approach and the subtle variability in clinical decision
making warrants periodical scrutiny of treatment profiles.
A study by Patil et al., showed that most of the colorectal cancers
presents at a younger age, with an aggressive histopathology, and with
involvement of both rectum and anal canal (5). There have been only few
epidemiological studies with respect to clinical outcomes of rectal cancer in
India and with its wide cultural and socio-ethnic differences, it is imperative
to assess the demographic and clinical profile that helps in strategic
planning, early diagnosis and efficient treatment modalities. Hence this study
aims to assess the clinical outcomes of patients with rectal cancer treated in
a tertiary care centre in South India.
Materials and methods
A total of 131 patients were diagnosed and
treated from June 1st 2014 to May 31st 2015 in a tertiary
care hospital in South India. This is a retrospective analytical study with
data obtained from the Medical Records in the hospital. It was based on content
analysis where the clinical and histopathological staging, treatment and follow up
details were collected from the case records using a structured proforma.
All rectal carcinoma patients with histology
of adenocarcinoma during the study period were included in the study. Histology
other than adenocarcinoma were excluded from the study. Informed consent was
obtained from all patients and the study was approved by Institutional Review
Board (No: 09/2016/04).
Statistical analysis
All patients (n = 131) were analysed and
follow up data was updated until April 2023. The primary endpoints analysed
were disease free survival (DFS) and overall survival (OS). DFS was defined as
the period from the date of registration to the date of locoregional relapse,
distant relapse or death, whichever occurred earlier. OS was defined as the
period from the date of registration to the date of death from any cause. The
statistical analysis was done using SPSS version 20. The data was analysed and
results were tabulated using descriptive statistics. Continuous variables were expressed as
mean and standard deviation, and categorical variables as counts and
percentages. Survival curves were
generated using the Kaplan‑Meier method and statistical significance was
assessed using the log‑rank test. The risk for survival was assessed
using cox regression analysis and a p-value < 0.05 was considered significant.
Results
Baseline characteristics
From June 1st 2014 to May 31st
2015, 131 patients with biopsy proven rectal adenocarcinoma were analysed in
the present study. Baseline characteristics are shown in table 1. The median
age of the population was 59 years ranging from 24 to 91 years. There were 82
males (62.59%) and 49 females (37.41%) included in the study. Mid rectal cancer
(defined as tumors residing 5-10cm from anal verge)
was the most common occurring in 51 patients (38.93%) closely followed by lower
rectal cancer (defined as tumors residing <5cm
from anal verge) diagnosed in 45 patients (34.36%) and upper rectal cancer
(defined as tumors residing 10-15cm from anal verge)
in 35 patients (26.71%). Majority had cT3 disease (38.16%) followed by cT2
(30.55%) and cT4 disease (16.03%). With respect to nodal status, 72 patients
(54.96%) had node negative disease. Stage II and Stage III disease dominated
the study population each contributing to 32.82% followed by stage I disease
(27.49%).
High risk factors like obstruction,
perforation, presence of LVE (lymphovascular
emboli)/PNI (Perineural invasion) and inadequate nodal sampling, were present
in 11 patients (8.39%). High-risk pathology like poorly differentiated
carcinoma (2.29%), mucinous adenocarcinoma (12.21%) and presence of signet ring
cells (9.92%) were observed.
Treatment characteristics
Prior to reporting at our centre, 32 patients
(24.42%) received some form of oncological treatment elsewhere. Of the
remaining 99 patients, 82 patients (82.82%) received neoadjuvant
chemoradiation. Pathological Complete Response was seen in 8.39% patients.
Pathologically node-negative disease was observed in 52.7% of patients. About
88 patients received adjuvant chemotherapy, of them 75% patients were treated
with mFOLFOX-6 (5-Fluorouracil + Folinic acid + Oxaliplatin) and 14.7% patients
were treated with CAPOX (Capecitabine + Oxaliplatin) regimen.
Table 1. Baseline Characteristics.
|
Characteristics |
Number (n) |
Percentage (%) |
|
Age |
||
|
<40 |
10 |
7.6% |
|
40-59 |
61 |
46.56% |
|
60-79 |
55 |
42.04% |
|
>80 |
5 |
3.8% |
|
Sex |
||
|
Male |
82 |
62.59% |
|
Female |
49 |
37.41% |
|
Site of disease |
||
|
Upper |
35 |
26.71% |
|
Mid |
51 |
38.93% |
|
Lower |
45 |
34.36% |
|
Tumour characteristics |
||
|
T1 |
20 |
15.26% |
|
T2 |
40 |
30.55% |
|
T3 |
50 |
38.16% |
|
T4 |
21 |
16.03% |
|
Nodal Status |
||
|
N0 |
72 |
54.96% |
|
N1 |
33 |
25.20% |
|
N2 |
26 |
19.84% |
|
Stage of the disease |
||
|
Stage I |
36 |
27.49% |
|
Stage II |
43 |
32.82% |
|
Stage III |
43 |
32.82% |
|
Stage IV |
9 |
6.87% |
|
High risk factors |
||
|
Obstruction |
1 |
0.76% |
|
Perforation |
1 |
0.76% |
|
LVE/PNI |
4 |
3.05% |
|
Inadequate Lymph Node sampling |
5 |
3.81% |
|
Histology |
||
|
Well Differentiated |
21 |
16.04% |
|
Moderately Differentiated |
78 |
59.54% |
|
Poorly differentiated |
3 |
2.29% |
|
Mucinous |
16 |
12.21% |
|
Signet ring cell |
13 |
9.92% |
|
Radiotherapy |
||
|
NACTRT |
83 |
63.35% |
|
Adjuvant CRT |
17 |
12.97% |
|
SCRT |
3 |
2.22% |
|
Palliative RT |
6 |
4.58% |
Abbreviations:
LVE - lymphovascular emboli; PNI - Perineural
invasion; NACTRT – Neoadjuvant chemoradiotherapy; CRT – chemoradiotherapy; SCRT
– short course radiotherapy; RT – Radiotherapy.
The median follow up was 105.8 months (16.6
to 182.6 months). The 8-year OS and DFS was shown to be 77.2% and 78.8%
respectively. Figure 1 shows the Kaplan Meier curves for 8-year DFS and OS in
our study.
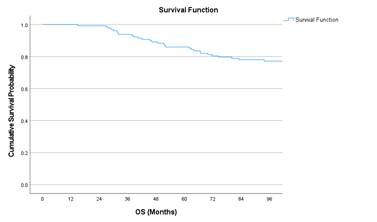
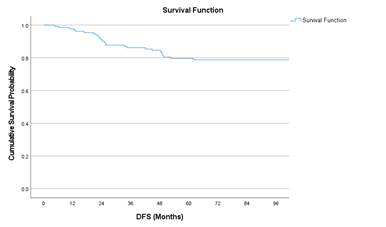
Figure 1. Kaplan Meier
curves of 8-year DFS and OS.
Tables 2 and 3 show the cumulative survival
probabilities of OS and DFS with respect to stage, sex, site of disease,
histology, and various chemotherapy regimens respectively. Figure 2 shows the
Kaplan Meier curves of OS with respect to stage, histology and nodal status.
Table 2. Survival probability with respect to OS.
|
Variables |
Cumulative Survival Probability (%) |
P-value |
|
|
Stage |
I |
77.1 |
0.009 |
|
II |
90.1 |
||
|
III |
71.9 |
||
|
IV |
44.4 |
||
|
Sex |
Female |
70.5 |
0.101 |
|
Male |
81.1 |
||
|
Age |
<50 |
72.1 |
0.371 |
|
>=50 |
78.9 |
||
|
Site |
Lower |
81.0 |
0.756 |
|
Mid |
76.2 |
||
|
Upper |
74.1 |
||
|
Histology |
MD |
76.2 |
<0.001 |
|
PD |
0.0 (all
died) |
||
|
WD |
84.8 |
||
|
Prior
Treatment |
Treated
elsewhere |
84.4 |
0.258 |
|
No Treatment |
74.7 |
||
|
Pathological
Nodal Status |
Negative |
83.6 |
0.063 |
|
Positive |
68.3 |
||
|
Adjuvant
Chemotherapy |
CAPECITABINE |
100.0 |
0.043 |
|
CAPOX |
61.5 |
||
|
FOLFOX |
81.4 |
||
|
Palliative
Chemotherapy |
CAPOX |
66.7 |
0.007 |
|
FOLFOX |
0.0 (all
died) |
||
|
5-FU+LV |
100.0 |
||
Abbreviations:
MD- Moderately Differentiated; WD- Well Differentiated; PD- Poorly
Differentiated; 5FU + LV – 5-Flourouracil + Leucovorin; mFOLFOX-6
(5-Fluorouracil + Folinic acid + Oxaliplatin); CAPOX (Capecitabine +
Oxaliplatin).
Table 3. Survival probability with respect to DFS.
|
Variables |
Cumulative Survival Probability (%) |
P-value |
|
|
Stage |
I |
80.3 |
0.775 |
|
II |
80.8 |
||
|
III |
73.7 |
||
|
IV |
88.9 |
||
|
Sex |
Female |
73.0 |
0.116 |
|
Male |
82.4 |
||
|
Age |
<50 |
75.4 |
0.434 |
|
>=50 |
80.0 |
||
|
Site |
Lower |
75.0 |
0.592 |
|
Mid |
77.3 |
||
|
Upper |
85.7 |
||
|
Histology |
MD |
73.8 |
0.391 |
|
PD |
66.7 |
||
|
WD |
85.7 |
||
|
Prior
Treatment |
Treated
elsewhere |
77.6 |
0.928 |
|
No Treatment |
79.2 |
||
|
Pathological
Nodal Status |
Negative |
75.0 |
0.473 |
|
Positive |
81.7 |
||
|
Adjuvant
Chemotherapy |
CAPECITABINE |
77.8 |
0.904 |
|
CAPOX |
76.2 |
||
|
FOLFOX |
80.2 |
||
|
Palliative
Chemotherapy |
CAPOX |
66.7 |
0.459 |
|
FOLFOX |
0.0 (all
died) |
||
|
5-FU+LV |
100.0 |
||
Abbreviations:
MD- Moderately Differentiated; WD- Well Differentiated; PD- Poorly
Differentiated; 5FU + LV – 5-Flourouracil + Leucovorin; mFOLFOX-6
(5-Fluorouracil + Folinic acid + Oxaliplatin); CAPOX (Capecitabine +
Oxaliplatin).
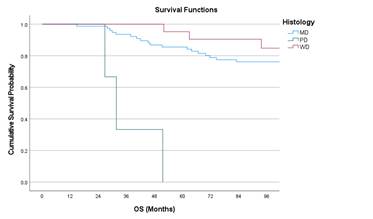
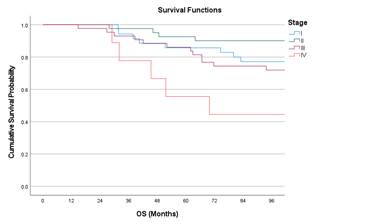
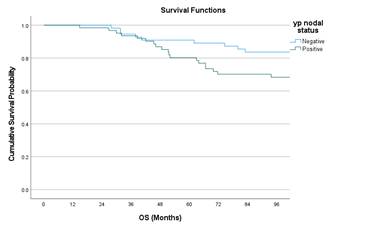
Figure 2. Kaplan Meir curves of OS with respect to Stage, Nodal status, and
histology
Univariate analysis showed stage IV had worse
outcomes compared to stage I (Hazard ratio (HR) 3.19; p = 0.042) and poorly
differentiated adenocarcinoma had worse OS compared to moderately
differentiated adenocarcinoma (HR 14.94; p = <0.001). Multivariate cox
regression analysis showed poorly differentiated histology is associated with
poor OS with an HR 9.47 (p = 0.003). Table 4 and 5 shows the univariate
analysis with respect to OS and DFS respectively. Table 6 shows the multiple
cox regression analysis affecting OS.
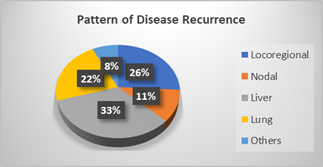
Figure 3 shows the pattern of recurrence
after radical treatment with locoregional recurrence of 26% and among the
distant sites, liver metastasis contributes to 33% followed by lung (22%),
non-regional nodes (11%) and other sites (8%) which includes bone, brain and
adrenals.
Discussion
Colorectal cancer has witnessed a staggering rise in its incidence and
parallelly the evolution of surgical and chemoradiation techniques have
improved the survival of the patients. In general, women tend to have lower
incidence and higher survival in colorectal cancer (6). Losurdo et al.,
published a gender focussed analysis of long-term outcomes of colorectal cancer
that revealed a higher 5-year (86.9% vs 80.5%) and 10-year survival (80% vs
73.3%) for women compared to men (7). This contrasts with our study where no
statistically significant survival differences were seen between men and women.
Siegel et al., reported rising incidence rates of colorectal cancer in
younger population (less than 50 years) in western countries over the last 2
decades (8). A retrospective analysis in an Indian population showed that
around 21% of patients with colorectal cancer were less than 40 years (9). Hong
et al., showed that the lowest 5-year OS and DFS were seen in age group less
than 40 years (62.5% and 52.1% respectively) (10), similar to our study where a
statistically non-significant trend of lower survival rates were seen in
patients less than 50 years.