Nitric oxide (NO) is a small molecule with a short half-life and
lipophilic properties, and many living cells can to make this molecule (1). In mammals, the production and formation of this
molecule vary among different species. In Wistar rats, the production of nitric
oxide is reported to be between 0.85-0.33 µmol/kg/h (2). The molecule has a very short half-life in the body,
measuring less than 0.1 seconds (3).
The genus Satureja (family Lamiaceae) contains over 200
species, with several endemic to mountainous areas of Iran. Various biological
activities have been reported for Satureja species, including
antimicrobial, anti-inflammatory, and antioxidant effects (4). Nitric oxide
(NO) is a small, short-lived, lipophilic molecule produced by many cell types.
NO plays essential roles in vascular homeostasis, including vasodilation, inhibition
of platelet aggregation, and regulation of blood pressure. Excessive NO production
occurs in pathological conditions such as hypertension, atherosclerosis, septic
shock, and ischemia (5-8). Among the biological processes in which NO plays a
role are the regulation of reproductive actions (8), lipolysis, and regulation
of energy balance (9). However, there is limited scientific evidence on the
impact of Satureja mutica extract on NO modulation, particularly in
vivo. This study addresses this gap by evaluating the extract’s effect of S.mutica
on serum NO levels in rats and identifying its major phenolic constituents.
Therefore, this study was conducted in response to the abundance of evidence in
Guilan province's traditional medicine indicating that regular use of Satureja
mutica can enhance heart function.
Materials and methods
Samples
The present study follows an experimental
laboratory approach, where samples under treatment are compared to a control
group. Male Wistar
rats, which were adults and weighed 190 ± 10 grams, were sourced from the
Pasteur Institute of Iran. The rats were housed in a special animal room at a
temperature of 25°C±2°C degrees Celsius, with a
12-hour light and 12-hour dark cycle.
Animals Grouping
Animals were randomly assigned to three groups
(n=5 each): (1) control (no treatment), (2) saline group (intraperitoneal
normal saline), and (3) treatment group (S. mutica extract 100
mg/kg/day, IP, for 7 days). They had access to unlimited food and water, which
provided in the form of ready-made mouse feed from Pars Animal Factory. The
rats randomly divided into groups, and each group was assigned a number for
identification. The rats were adapted to the presence of the researcher. All
procedures complied with the Institutional Animal Care and Use Committee
guidelines at Guilan University of Medical Sciences. Animals were humanely
euthanized under anesthesia before blood collection.
Extract preparation and administration
The botanist Dr. Mahdavi collected and
identified the S. mutica plant in the vicinity of Rostam Abad city in
Guilan province. The plant was given the code Herbarium 7011 and then taken to
the Herbarium at the Guilan Agricultural Education and Natural Resources
Research Center. Initially, the plant's leaves were cleaned before being left
to dry in the shade for a week. Subsequently, the dried leaves were ground into
powder using an electric mill, then the powder was dissolved in 80% ethanol.
Following filtration of the solution, a rotary machine was utilized to separate
the solvent from the extract. Finally, the extract was dried and an aqueous
solution was obtained by adding normal saline. The intraperitoneal injections
of the extract were administered once daily at 10 am for a duration of 7 days.
Upon finishing the experiments at the end of a week, blood samples were
obtained from the animals. The animals were anesthetized with ether before
blood collection, and blood was drawn from the heart. Subsequently, serum was
separated from the blood samples using a routine method. The serum nitric oxide level was measured
through the spectrophotometry method.
Spectrophotometric analysis of nitric oxide and nitrites in biological
samples using the Griess reagent relies on a series of chemical reactions.
These reactions involve diazotization followed by coupling. The resulting azo
compound from Griess reactions exhibits absorbance in the ultraviolet and
visible spectrum, ranging from 300 to 700 nm (10).
HPLC-PDA separation
A new method using high-performance liquid
chromatography with a photodiode array detector (HPLC/PDA) was used to measure
phenolic compounds in extract of S. mutica accurately. HPLC-PDA was used
to examine organic compounds. HPLC separates these compounds by their
interaction with the stationary phase, while PDA measures absorbance at various
wavelengths, offering details on the quality and quantity of the compounds in
the sample.
Separation of phenolic compounds extracted
from Satureja mutica extract was performed by using an
ethanol/methanol/formic acid/water solution with HPLC-PDA, detected at 280 nm
(A) and 520 nm (B). The column used was Luna RP-C18(2) (250 × 2.0 mm I.D., 5
μm) with a C18 guard cartridge column (4 × 2.0 mm I.D.) from Phenomenex.
The compounds were eluted using a multi-segment linear gradient, with a flow
rate of 0.2 mL per minute.
Statistics
The statistical analysis was conducted using
the one-way analysis of variance (ANOVA) method in SPSS version 17. This
approach was selected to determine whether there were statistically significant
differences among the means of the different experimental groups. Following the
ANOVA test, Benferoni’s post hoc test was applied to perform multiple pairwise
comparisons between groups, thereby identifying the specific group differences
responsible for the overall statistical significance. Throughout the analysis,
a p-value of less than 0.05 (p < 0.05) was considered the threshold for
statistical significance, indicating that the observed differences were
unlikely to have occurred by random chance.
Results
Table 1 presents the nitric oxide levels in the analyzed groups. The
findings suggest that the nitric oxide levels in the group that was
administered normal saline did not exhibit a notable alteration in comparison
to the control group. However, there was a considerable reduction in nitric
oxide levels in the rats that were given the extract when contrasted with the
control group, only following the administration of Satureja mutica
extract (P<0.001).
Figure 1 shows peaks identification: Galic acid; 3.,4 dhb; Chlorogenic
acid; Cathechin Caffeic acid; Vanilic acid; 2.5 dhb; Syrginic acid; P-cumaric
acid; Ferrulic acid; Rutin; Salycilic acid; Rosmarinic acid; Cinamic acid;
Quercetin; Kaempferol and Apigenin.
The chromatogram (Figure 1) displays the separation and detection of
chemical compounds present in the S. mutica extract using
High-Performance Liquid Chromatography (HPLC) coupled with a Photodiode Array
(PDA) detector. Each peak represents a distinct compound, with the X-axis
indicating retention time (compound separation time) and the Y-axis showing
signal intensity (relative concentration). Based on the plant's phytochemical
profile, major peaks likely correspond to terpenes (e.g., carvacrol, thymol) and
phenolic compounds such as quercetin, rutin, or rosmarinic acid. The varying
peak heights and widths suggest differences in compound abundance and purity,
with sharper peaks indicating well-separated components. This HPLC-PDA analysis serves as a fingerprint for the
extract's chemical composition, highlighting bioactive phenolics like
quercetin, which may explain the biological activities observed in Figure 2.
The method's precision allows for qualitative identification of compounds,
though exact confirmation would require comparison with reference standards or
mass spectrometry (MS) data. The presence of these compounds underscores the
extract's potential pharmacological value, particularly in studies involving
antioxidant or anti-inflammatory effects. Further analysis could quantify
specific compounds and explore their synergistic interactions.
The information is presented as "mean ± standard deviation". P
values obtained from one-way analysis of variance are used to compare and
indicate differences from the control group.
The label NS indicates that there is no statistically significant
difference compared to the control group.
Table 1. NO serum level in the different groups following the administration
of Satureja mutica extract.
|
Group |
NO(SEM) (μmol/L) |
P value |
|
Control |
99.37 ± 9.72 |
- |
|
Normal Saline |
55.28 ± 6.2 |
NS |
|
Extract (100
mg/kg) |
44.12 ± 10.87 |
0.001>P |
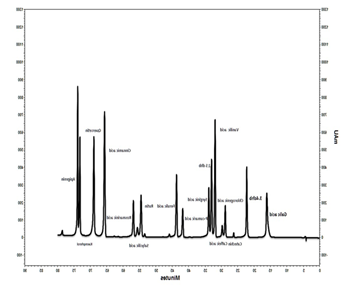
Figure 1. Chromatogram of Satureja mutica extract in HPLC-PDA.
Discussion
The Lamiaceae family consists of over 200 species in the genus Satureja
L., primarily found in the Mediterranean region. Among them, eight species are
unique to mountainous areas in Iran, especially Guilan provinve, north part of
Iran (11). The results demonstrated that there was no significant change in the
serum nitric oxide level in the group treated with normal saline compared to
the control group. This suggests that administering the extract through
injection did not impact the test outcomes. In contrast, it was found that the
serum nitric oxide level in rats treated with the hydroalcoholic extract of S.
mutica significantly decreased in comparison to the control group
(P<0.001).
Several studies have shown that the essential oil of Satureja
species contains high levels of monoterpenoids and phenolic compounds like
carvacrol, γ-terpinene, thymol, and p-cymene. The antimicrobial properties
of the essential oil and extract of particular Satureja species have
been documented (12). While extracts, bioactive fractions, or compounds derived
from medicinal plants serve various purposes, the techniques employed to obtain
them remain mostly consistent regardless of the intended biological testing.
The core steps in obtaining high-quality bioactive molecules include selecting
a suitable solvent, utilizing extraction methods, conducting phytochemical
screening procedures, employing fractionation methods, and utilizing
identification techniques. In this research, two frequently used polar solvents
(water and alcohols) were utilized for Soxhlet extraction (10).
Nitric oxide synthase (NOS) comprises three different isozymes that play
a role in producing NO: the constitutive endothelial (eNOS) and neuronal (nNOS)
isozymes, as well as the inducible isozyme. It is understood that the inducible
isozyme (iNOS) is present in various cell types, including cardiac myocytes.
iNOS is typically activated in response to a range of physiological and
pathophysiological triggers, such as vigorous exercise and hypoxia(13-14). Echinodorus
grandiflorus, also known as Burhead, is utilized in traditional Brazilian
medicine as a diuretic treatment. The herb stimulates prolonged urine
production and lowers blood pressure by interacting with muscarinic and
bradykinin receptors, affecting pathways related to prostaglandins and nitric
oxide (15). Stephania tetrandra can help control hypertension by
decreasing the expression of inducible nitric oxide synthase (iNOS) and
inhibiting Ca2+ channels. The alkaloid tetrandrine, found in this
plant, possesses anti-inflammatory and antioxidant properties that likely
contribute to its ability to lower blood pressure (16). The Tianma methanolic
extracts (at a concentration of 0.02 ml/g) demonstrated anti-inflammatory
effects by reducing iNOS expression and levels of NO (17).
The highest percentage of phenolic compounds in the Satureja mutica
extract was quercetin. Quercetin, a flavonoid present in various
fruits, vegetables, and grains, possesses potent antioxidant and
anti-inflammatory properties. Some research suggests that quercetin can boost
the activity of endothelial nitric oxide synthase (eNOS), the enzyme responsible
for generating nitric oxide in blood vessels, thereby assisting in improving
endothelial function and promoting vasodilation (13-14). Acting as a robust
antioxidant, quercetin could safeguard nitric oxide from degradation by
reactive oxygen species (ROS), preserving its availability and efficacy. The
results of this study probably suggest that if a high percentage of a plant's
polyphenolic compounds is quercetin, changes or effects on the cardiovascular
system can be expected. Nonetheless, further research is necessary to fully comprehend the extent
and mechanisms of quercetin's impact on nitric oxide and overall cardiovascular
function. Inhibition of nitric oxide
production can be achieved through pharmacological means, such as blocking NOS
activity or downstream signaling molecules. Pharmacological and
non-pharmacological approaches are not considered in this study.
Mechanisms by Which Quercetin May Decrease NO Levels
1)
Inhibition
of Inducible Nitric Oxide Synthase (iNOS)
In inflammatory conditions, quercetin has been shown to suppress the
expression of iNOS, an enzyme responsible for high-output NO production. This
suppression occurs through the inhibition of the NF-κB signaling pathway,
leading to reduced NO synthesis in activated immune cells .
2)
Suppression
of Endothelial Nitric Oxide Synthase (eNOS) Expression
Some studies suggest that quercetin can downregulate eNOS expression in
endothelial cells, particularly under pro-inflammatory stimuli like TNF-α.
This downregulation may result in a decrease in NO production, affecting
vascular tone and blood pressure regulation.
3)
Modulation
of Neuronal Nitric Oxide Synthase(nNOS)
Quercetin's effects on nNOS are less well-defined, but there is evidence
indicating that it may influence nNOS activity, potentially impacting NO levels
in neuronal tissues.
The observed upregulation of iNOS mRNA and protein by quercetin, coupled
with decreased NO production, holds significant implications for cancer
biology. iNOS-derived NO plays a dual role in cancer progression, acting as
either a pro-tumor or anti-tumor agent depending on concentration and context.
At high levels, NO can promote DNA damage and angiogenesis, fueling tumor
growth, while at low levels, it may suppress immune responses. Quercetin's
ability to modulate this balance suggests its potential as a chemopreventive
agent, particularly in cancers where chronic inflammation drives tumorigenesis,
such as colorectal or breast cancer. Targeting the iNOS/NO pathway with
phenolic compounds like quercetin could offer a strategic approach to cancer
therapy. By reducing excessive NO, quercetin may mitigate inflammation-induced
carcinogenesis while preserving anti-tumor immunity. However, the paradoxical
effects increased iNOS expression but decreased NO, warrant further
investigation to optimize dosing and avoid unintended pro-tumor effects.
Clinical studies are needed to validate these mechanisms in human models and
explore synergies with conventional therapies, potentially positioning
quercetin as an adjunct in precision oncology (Figure 2).