Metastatic
carcinoma-ex pleomorphic adenoma of the pharynx with carotid space invasion: a
rare case report
Siddharth
Arora 1*, Kirti Mohanty 1, Kriti Grover 1, Mansi
Dey 2, Sandeep Ramawat 1
1 Rohilkhand
Medical College and Hospital, Bareilly, Uttar Pradesh, India
2 Mahamana
Pandit Madan Mohan Malviya Cancer Centre, Varanasi, Uttar Pradesh, India
Corresponding Author: Siddharth
Arora
* Email: drsiddhartharora25@gmail.com
Abstract
Introduction: Carcinoma ex pleomorphic adenoma (CXPA) is a rare, aggressive malignancy
of the salivary glands that arises from a pre-existing pleomorphic adenoma.
Although pleomorphic adenomas are benign, their potential for malignant
transformation necessitates timely diagnosis and management. Pathological
assessment remains the gold standard for diagnosis, with surgery followed by
radiotherapy being the standard of care.
Case Presentation: A 38-year-old male presented with a metastatic carcinoma ex pleomorphic
adenoma of the pharynx, with invasion into the carotid space. Diagnosis was
confirmed histopathologically. The patient underwent
palliative radiotherapy.
Discussion: CXPA is often difficult to diagnose due to its overlapping features
with benign tumors, especially in atypical locations such as the pharynx.
Malignant transformation typically indicates a more aggressive clinical course,
including local invasion and distant spread. In this case, carotid space
involvement further complicated management, highlighting the importance of
early detection and comprehensive treatment.
Conclusion: Early recognition of pleomorphic adenomas and their potential for
malignant transformation is critical. This case emphasizes the need for a
multidisciplinary approach in diagnosing and managing rare presentations of
CXPA to improve patient outcomes.
Keywords: Carcinoma ex-pleomorphic adenoma, Recurrent pleomorphic adenoma,
Carotid space invasion
Salivary gland tumors are uncommon,
accounting for only 3 %–10 % of head and neck neoplasms. They can arise in the
major salivary glands (parotid, submandibular, and sublingual) or in the minor
salivary glands (small, predominantly mucus-secreting glands located beneath
the mucosal lining of the upper aerodigestive tract, such as labial, lingual,
palatal, buccal, glosso-palatal, and retromolar
glands) (1, 2).
Carcinoma ex-pleomorphic adenoma (CXPA)
is a carcinomatous transformation within a primary (de novo) or recurrent
pleomorphic adenoma of a salivary gland, most commonly found in the parotid
gland. However, it can also originate in the submandibular gland and rarely in
minor salivary glands located in the hard and soft palate. Tumors arising from
minor salivary glands are typically smaller in size compared to those from the
major salivary glands, and the incidence is less than 7%. In addition to
salivary glands, CXPA has also been identified in lacrimal glands, nasal
cavities, trachea, and breast (3, 4). Here, we present a case of recurrent
pleomorphic adenoma of the soft palate that ultimately transformed into CXPA of
the pharynx with carotid space invasion and distant metastasis.
Case presentation
A 38-year-old
male patient initially presented in his native country, Zambia, in June 2006
with a swelling on the left side of the soft palate. The swelling was excised,
and histopathology revealed pleomorphic adenoma (PA). However, he developed
recurrences in 2012, 2015, and 2016, each of which was surgically re-excised,
with histopathology confirming PA in all instances. In December 2018, he
presented with an extensive and inoperable local recurrence, and he underwent
palliative radiation therapy (30 Gy in 10 fractions over 2 weeks) in Zambia. He
was later referred to our center for further evaluation and treatment.
On local
examination, the patient exhibited a large, irregular lesion in the left oral
cavity, measuring approximately 6 × 5 cm. The mass extended into the right side
of the oral cavity, including the right tonsillar pillar, and inferiorly
involved the base of the tongue. Superiorly, the lesion extended to the soft
and hard palate. Laterally, it involved the retromolar space and left tonsillar
pillar. Palpation revealed enlarged left level IA and IB lymph nodes. The
patient had difficulty closing his mouth and complained of mid-back pain, which
required analgesic medication. Neurological examination was unremarkable.
A whole-body
PET-CT was performed, revealing a large, necrotic mass involving the left
pharyngeal space, obliterating the posterior nasopharynx, and extending into
the supraglottic region with luminal obstruction. Superiorly, the mass extended
into the left infratemporal fossa and carotid space. Posteriorly, the lesion
thinned the wall of the left maxillary sinus, medially indenting the soft
palate and the posterior one-third of the tongue. The lesion showed moderate to
intense heterogeneous uptake (SUVmax: 14.6).
Bilateral sub-centimeter nodules were seen in the lungs with minimal FDG uptake
(SUVmax: 3.8), consistent with bilateral lung
metastasis. Additionally, FDG-avid lytic lesions were observed in the D11 and
L3 vertebrae with soft tissue involvement (SUVmax:
9.5 in L3 vertebra) (Figure 1).
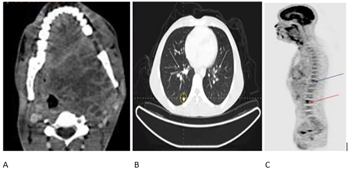
Figure 1. PET CT: A:
Axial view of PET scan showing extent of the primary tumor; B: Axial view of
PET scan showing distant metastasis to bilateral lungs; C: Sagittal view of PET
scan showing lytic lesions at D11 (blue arrow) and L3 (red arrow).
Histopathological
findings from a biopsy of the left retromolar trigone were consistent with CXPA
(Figure 2).
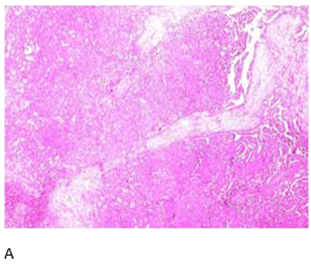
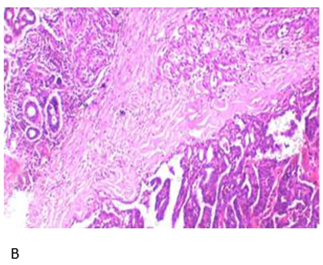
Figure 2. Hematoxylin
and Eosin:A: Low-power view
of salivary gland tissue infiltration by atypical ductal cells forming glands.
Cribriform pattern present; B: High-power view showing capsular invasion by
atypical ductal cells.
Immunohistochemistry
(IHC) findings showed that GFAP was positive in tubules with scattered focal
loss. HMWCK, p63, vimentin, and CK7 were diffusely positive. S100 stained
myoepithelial cells. The MIB1 index was 15-20% on hot spots. Her2-neu was
negative. myoepithelial cells. The MIB-1 index was 15-20 % on hot spots.
Her2/neu was negative. Additionally, the left level IB lymph node biopsy showed
features consistent with a chondroid-forming myoepithelial-rich lesion,
favoring a recurrence of pleomorphic adenoma with extensive myoepithelial
areas.
Given the
extensive local recurrence, metastasis, and overall disease burden, surgery was
not considered feasible. Radiation therapy to the primary site (oropharynx) was
not offered due to the short intervals between prior treatments. The patient
was therefore planned for stereotactic body radiotherapy (SBRT) to treat the
symptomatic metastatic vertebral lesions at D11 and L3. SBRT with 6 MV photons
was administered to the D11 vertebra to a total dose of 27 Gy in 3 fractions (9
Gy per fraction) and to the L3 vertebra to a total dose of 18 Gy in a single
fraction. After three cycles of chemotherapy with paclitaxel and carboplatin
and SBRT, the patient showed stable disease on follow-up PET-CT imaging. He
reported symptomatic improvement, including pain relief, during a short 6-month
follow-up period. However, the patient did not return for further follow-up after
this period.
Discussion
CXPA accounts for approximately 12 % of
all salivary carcinomas. The peak incidence occurs in the 6th to 7th decade of
life (about 1-2 decades later than pleomorphic adenoma). While most are now
recognized as salivary duct carcinomas, other morphological subtypes, including
myoepithelial carcinoma and epithelial-myoepithelial carcinoma, are also seen
(5). Microscopically, CXPA presents with varying degrees of invasion, including
intracapsular, minor extracapsular (<5 mm beyond the capsule), and wide extracapsular
(>5 mm beyond the capsule) invasion (6). Metastatic salivary gland tumors
are rare clinical findings, with only 20 % of patients with parotid gland
malignancy developing metastatic disease. Common sites for distant metastasis
include the lungs, bones, liver, and central nervous system, although
metastasis to the breast, ileum, spleen, and iliac crest has also been
described (7). Tumors with wide extracapsular invasion (beyond 5 mm of the
capsule) have a high risk of recurrence and distant metastasis, as seen in our
case, where symptomatic vertebral metastatic lesions developed at the D11 and
L3 vertebrae.
The transformation of PA to CXPA has been
widely recognized, with previous reports detailing similar patterns of
progression. In our case, PA occurred in a male patient at 38 years of age and
transformed into CXPA at 50 years of age. This was a recurrent PA of the soft
palate that ultimately transformed into myoepithelial CXPA of the pharynx over
a 12-year period. A case report of soft palate CXPA presenting as direct
cavernous sinus invasion has been reported in the literature (4). However, in
our patient, the CXPA of the pharynx invaded the carotid space, which, to our
knowledge, has not been reported before. The carotid space is a paired area
confined by the carotid sheath, a connective tissue boundary in the neck, which
extends from the jugular foramen at the skull base to the aortic arch at the
thoracic inlet. Lesions in the carotid space can arise from various structures
within the space, including the carotid artery, cranial nerves IX, X, and XII,
the ansa cervicalis, or the sympathetic plexus. These
lesions include paragangliomas, carotid body tumors, glomus jugulare,
glomus vagale, nerve sheath tumors, neurofibromas,
schwannomas, lipomas, and carotid sheath meningiomas (8).
Surgical resection is the most common
treatment for salivary gland tumors, and the extent of surgery is mainly
determined by anatomical and clinical factors. However, for some lesions, the
correct diagnosis should guide therapeutic decisions. In the case of PA, the
risk of recurrence is about 2–3 %, with the highest risk seen in the myxoid
subtype and in the presence of an incomplete tumor capsule, pseudopodia, and
satellite nodules. Thus, more extended surgical techniques are preferred.
Another factor necessitating extended surgery is recurrence (9). Complete
resection with an intact capsule results in a lower
recurrence rate. A higher number of recurrences significantly increases the
risk of subsequent recurrence. PA is not associated with age or gender, and
unlike Warthin tumor, it is not associated with tobacco use (10).
For patients with recurrent PA,
radiotherapy is associated with a significant reduction in the risk of
recurrence compared to surgery alone, regardless of the completeness of
resection. Although concerns about radiation-associated toxicities exist, they are
generally limited and do not outweigh the potential benefits of radiotherapy
for recurrence. In this case, the patient did not receive adjuvant radiotherapy
when he was operated on for recurrent PA, leading to multiple recurrences. This
ultimately resulted in transformation into CXPA, with the disease becoming
unresectable and distant metastasis developing. The patient was subsequently
offered SBRT to symptomatic bony metastatic sites and palliative chemotherapy.
However, the patient was lost to follow-up after 6 months, a key limitation of
this case report. This early loss limits the ability to assess the long-term
effectiveness of these therapies and their impact on the patient's quality of
life.
The available literature on the role of
chemotherapy in this context is limited. However, some chemotherapeutic
agents—such as platinum compounds (cisplatin, carboplatin), taxanes
(paclitaxel), and alkylating agents (cyclophosphamide, fluorouracil)—have been
used in metastatic settings with varying results. Adjuvant concurrent
chemotherapy is generally recommended in cases with high-risk features,
including unresectability, positive surgical margins,
or lymph node involvement.
There is growing interest in exploring
novel treatment strategies. Several investigational studies have focused on
molecular markers, particularly BRCA1 and BRCA2 mutations. In addition,
researchers have investigated a range of other genetic alterations, such as
HMGA2, CASP8, MLH1, RARB, KLK3, AI69125, EWSR1
rearrangement, and EGFR amplification. Despite these efforts, no
specific genetic alteration has yet been definitively linked to CXPA, and
targeted therapies for this condition remain under investigation.
Conclusion
Author contribution
Conceptualization, SA, KG and KM; writing original draft
preparation, SA, KG, KM and MD; writing, review and editing, SA, MD and SR;
supervision, KM and SA; Figures: KG and MD All authors have read the final
manuscript
Funding
There
is no funding.
Conflicts
of interest
There
are no conflicts of interest.
References
1. Spiro RH. Salivary neoplasms: Overview of a 35-year experience
with 2,807 patients. Head & Neck Surgery. 1986 Jan;8(3):177–84.
2. Kessler AT, Bhatt AA. Review of the Major and Minor Salivary
Glands, Part 1: Anatomy, Infectious, and Inflammatory Processes. Journal of
Clinical Imaging Science. 2018 Nov 15;8:47.
3. Khanna D, Chaubal T, Bapat R, Abdulla
AM, Philip ST, Arora S. Carcinoma ex pleomorphic adenoma: a case report and
review of literature. African Health Sciences. 1970 Jan 1;19(4):3253–63.
4. Chen HH, Lee LY, Chin SC, Chen I-How, Liao CT, Huang SF.
Carcinoma ex pleomorphic adenoma of soft palate with cavernous sinus invasion.
World Journal of Surgical Oncology. 2010 Mar 30;8(1).
5. Seethala RR, Stenman G. Update from
the 4th Edition of the World Health Organization Classification of Head and
Neck Tumours: Tumors of the Salivary Gland. Head and
Neck Pathology [Internet]. 2017 Feb 28;11(1):55–67.
6. Young A, Okuyemi OT. Malignant
Salivary Gland Tumors. 2023 Jan 12. In: StatPearls
[Internet]. Treasure Island (FL): StatPearls
Publishing; 2025 Jan–. PMID: 33079504.
7. Smith BM, Azouz V, Liu L, Williams G. Parotid adenocarcinoma
metastasis to the breast: a case report. J Surg Case Rep. 2020 Jul 2;2020(6):rjaa163.
8. Chengazi HU, Bhatt AA. Pathology of
the carotid space. Insights Imaging. 2019 Feb 15;10(1):21.
9. Michał Żurek, Fus Ł, Niemczyk K, Rzepakowska
A. Salivary gland pathologies: evolution in classification and association with
unique genetic alterations. European Archives of Oto-Rhino-Laryngology. 2023
Jul 13;280(11):4739–50.
10. Nicholas SE, Fu W, Liang A, DeLuna R, Luka Vujaskovic, Bishop
JA, et al. Radiation Therapy After Surgical Resection Improves Outcomes for
Patients With Recurrent Pleomorphic Adenoma. Advances
in Radiation Oncology. 2021 May 1;6(3):100674–4.