Incidence and
management of chemotherapy-related local complications in cancer patients in
Conakry, Guinea
Ibrahima
Kalil Condé 1*, Kalil Cissé
1, Abdoulaye
Mabinty Camara 1, Bangaly Traoré 1
1 Oncology Department,
Donka University Hospital, Conakry, Guinea
Corresponding Authors: Ibrahima
Kalil Condé
* Email: condekalil1800@gmail.com
Abstract
Introduction: Chemotherapy-related local complications (CRLC), such as phlebitis and
extravasation, can significantly affect patient quality of life and disrupt
treatment continuity. These complications are poorly documented in sub-Saharan
Africa, where structural and organizational constraints may contribute to
increased incidence and severity. This study aimed to determine the incidence,
characteristics, and associated factors of CRLC in an oncology unit in Guinea.
Materials and methods: A prospective descriptive and analytical study was conducted at
the Oncology Department of Donka University Hospital, Guinea, from November
2020 to February 2021. Patients with histologically confirmed cancers receiving
intravenous chemotherapy were included. Two groups were compared: patients with
and without CRLC. Sociodemographic, clinical, and therapeutic data were
analyzed using appropriate statistical tests.
Results: Among 88 patients (84.1% female; mean age 45.8 ± 16.7 years), 31
(35.2%) developed at least one CRLC. Out of 193 chemotherapy cycles, 51 CRLC
episodes (26.4%) were recorded, including phlebitis (15.0%) and extravasation
(11.4%). Most frequent protocols were doxorubicin + cyclophosphamide (AC) and
epirubicin + cyclophosphamide (EC), accounting for 33.0% of cases, followed by
docetaxel monotherapy (25%). CRLCs occurred during the first four cycles
(45.2%), predominantly grade 2 (82.4%), with favorable outcomes within 10 days
(96.1%). Peripheral venous access was used almost exclusively (100% with CRLC
vs. 96.5% without CRLC, p = 0.291). No statistically significant predictive
factor was identified. In 9.1% of cases, delayed consultation caused extensive
lesions requiring surgical excision, leading to a temporary chemotherapy
interruption without permanent functional sequelae.
Conclusion: CRLCs are frequent in our resource-limited setting, affecting
more than one-third of patients and one-quarter of chemotherapy cycles.
Phlebitis and extravasation occurred mainly during the first cycles, with most
events being moderate but some requiring surgical management. These findings
highlight the urgent need to strengthen prevention strategies, staff training,
and access to appropriate vascular devices in order to reduce their incidence
and ensure treatment continuity.
Keywords: Chemotherapy, Extravasation, Phlebitis, Local toxicity, Sub-Saharan
Africa
Introduction
Cancer represents a major global public health challenge, with an
increasing incidence. Approximately 19 million new cases are diagnosed annually
worldwide (1). In Guinea,
Globocan estimates around 8,700 new cancer cases per year (2).
Following
histological confirmation, treatment strategies depend on tumor type and stage,
including chemotherapy, surgery, radiotherapy, or targeted therapies (3). Chemotherapy
remains central to cancer treatment, involving cytotoxic agents administered
alone or in combination for curative or palliative purposes. Common routes of
administration include oral, intravenous, intramuscular, subcutaneous,
intrathecal, intraperitoneal, intra-arterial, or topical, with intravenous
administration being the most common, usually via peripheral veins in the arm
or hand. In high-resource settings, chemotherapy may be administered via
central venous catheters or implantable ports, often combined with infusion
pumps, ensuring controlled flow (4).
Chemotherapy
exposes patients to multiple adverse effects, including local complications at
the infusion site, such as extravasation, phlebitis, and, in severe cases,
tissue necrosis (5–7). These events
can cause intense pain, lead to treatment interruption or delay, and
occasionally result in functional sequelae (8).
Estimating CRLC
incidence is challenging due to underreporting and the lack of centralized
registries. Reported extravasation rates range from 0.1–6% for peripheral lines
and 0.3–4.7% for central catheters (9,10). Data on
chemotherapy-induced phlebitis are highly heterogeneous, ranging from 3% to
89%, depending on diagnostic criteria and methodology (11).
Several risk
factors for CRLC have been identified, with extravasation risk depending on
patient factors (fragile or sclerosed veins, obesity, prolonged infusion) and
procedural factors (inexperienced staff, multiple punctures, bolus injections) (9). A UK study of
263 women treated with peripheral anthracyclines identified severe phlebitis as
associated with repeated use of the same arm, younger age, high doses,
comorbidities, injection pain, and cumulative cycles (12).
Although these
complications are well described in high-income countries, they pose an even
greater challenge in sub-Saharan Africa, where health systems face significant
infrastructure, human resources, and medical device limitations (13). In Guinea,
oncology care is primarily provided at Donka University Hospital in Conakry.
This center faces many challenges, including shortages of central venous
catheters, lack of specific antidotes such as dexrazoxane (used in
anthracycline extravasation), insufficient trained personnel, and frequent
delays in complication management. The absence of standardized protocols and
the frequent use of inappropriate vascular access devices increase the
morbidity risk from these adverse events. Despite these challenges, no local
study has documented to date the extent, clinical manifestations, or associated
factors of complications related to the intravenous administration of cytotoxic
treatments.
In this
context, it has become crucial to deepen the understanding of these
complications and to identify strategies for improving their management. This
study aims to analyze the incidence, clinical profiles, and risk factors of
CRLC in cancer patients in Conakry, Guinea.
Materials and methods
Study setting, design, and period
This was a prospective descriptive and analytical study conducted over
three months, from November 15, 2020, to February 15, 2021, at the Oncology
Department of Donka University Hospital (Guinea).
Study population and selection criteria
All patients with histologically confirmed cancer receiving
chemotherapy during the study period were included. Patients with pre-existing
CRLC or who did not consent were excluded.
Sample size justification
The sample size was not predetermined by statistical calculation.
Instead, all eligible patients treated with intravenous chemotherapy during the
three-month study period were consecutively included. This exhaustive
recruitment allowed us to capture the full range of CRLC occurring in routine
practice in our setting.
Variables collected
Collected variables included:
·
Sociodemographic: age, sex, comorbidities;
·
Clinical:
cancer type, stage;
·
Therapeutic:
chemotherapy protocol, administration route, puncture attempts, catheter gauge,
cycle number;
·
CRLC:
type, grade according to the Common Terminology Criteria for Adverse Events
(CTCAE v5.0), time to onset, evolution;
·
Corrective and preventive measures.
The CRLC diagnosis was based on clinical
examination of the injection site. Patients were evaluated on infusion day and
at each subsequent cycle. In case of complaints, patients contacted the team or
presented to the hospital, where assessment focused on general condition and
injection site. No additional imaging (Doppler ultrasound, thermography) was
performed, as thermography was unavailable in Guinea.
Data analysis
Data were analyzed using SPSS software
(version 21). Qualitative variables were expressed as frequencies and
percentages, and quantitative variables as means ± standard deviation.
CRLC incidence was calculated using two
approaches:
-
Patient-based
incidence: proportion of patients experiencing ≥1 episode during the
study period;
-
Cycle-based
incidence: proportion of chemotherapy cycles complicated by CRLC.
Frequencies of specific complications
(phlebitis, extravasation) were expressed as percentages of total cycles and
relative proportions among all episodes.
For comparison, patients were divided into two
groups: with CRLC and without CRLC. Factors associated with CRLC occurrence
were analyzed using Chi-square or Fisher’s exact tests for qualitative
variables and t-test or Mann–Whitney test for quantitative variables. A p-value
< 0.05 was considered statistically significant.
Ethical considerations
The study was conducted following the principles of the Declaration of
Helsinki. The protocol was reviewed and approved by the scientific committee of
the Oncology Department of Donka University Hospital. All data were anonymized
and treated confidentially. Written informed consent was obtained from patients
at the time of initial care and recorded in their medical files.
Results
Frequency and
distribution of local complications
Among 88 patients included, 31 (35.2%) experienced at least one CRLC
episode during follow-up, while 57 (64.8%) did not experience any. A total of
193 chemotherapy cycles were administered, of which 51 (26.4%) were complicated
by CRLC. Among these episodes, 29 were phlebitis (15.0% of cycles) and 22 were
extravasations (11.4% of cycles). Phlebitis accounted for 56.9% of all recorded
episodes.
Sociodemographic
characteristics
The mean age was 45.8 ± 16.7 years (range: 4–81 years). Females
predominated (84.1%), with a higher proportion in the CRLC group (94.0%) than
the non-CRLC group (79.0%) (p = 0.074). The most frequent comorbidities were
hypertension (25.8% with CRLC vs. 12.2% without CRLC) and obesity (58.1% vs.
38.7%), without significant differences (Table 1).
Table 1. Sociodemographic characteristics according to
the occurrence of CRLC.
|
Characteristics
|
CRCL
|
Without CRCL
|
Total
|
p-
value
|
|
Mean age (years)
|
44.1±16.2
|
46.7±13.4
|
45.8±16.6
|
0.835
|
|
Sex
|
|
|
|
|
|
Female
|
29 (94.0 %)
|
45 (79.0 %)
|
74 (84.1 %)
|
0.074
|
|
Male
|
2 (6.4 %)
|
12 (21.0 %)
|
14 (15.9 %)
|
|
Comorbidities
|
|
|
|
0.556
|
|
Hypertension
|
8 (25.8 %)
|
7 (12.2 %)
|
15 (17 %)
|
|
Diabètes
|
3 (9.7 %)
|
3 (9.7 %)
|
6 (6.8 %)
|
|
Obesity/
Overweight
|
18 (58.1 %)
|
12 (38.7 %)
|
30 (34.1 %)
|
|
HIV
|
1 (3.2 %)
|
-
|
1 (1.1 %)
|
Clinical and
therapeutic characteristics
Breast cancer was the most common tumor site in both groups (64.5% with
CRLC vs. 53.0% without CRLC, p = 0.283). Most patients were locally advanced
and treated primarily with curative chemotherapy, without a significant
difference between groups (77.4% with CRLC vs. 79.0% without CRLC; p = 0.091)
(Table 2).
The most frequently administered protocols were AC and EC, accounting
for 33.0% of cases, followed by docetaxel monotherapy (25.0%), with no
significant difference between groups. Peripheral venous access was used almost
exclusively (100% with CRLC vs. 96.5% without CRLC, p = 0.291). Fewer than four
cycles were administered in 45.2% of patients with CRLC versus 56.1% without
CRLC (p = 0.332) (Table 3).
Table 2. Clinical characteristics according to the occurrence of CRLC.
|
Characteristics
|
CRCL
|
Without CRCL
|
Total
|
p-value
|
|
Primary site
|
|
|
|
|
|
Breast
|
20 (64.5 %)
|
30 (53.0 %)
|
50 (56.8 %)
|
0.280
|
|
Digestive
|
7 (22.6 %)
|
4 (7.0 %)
|
11 (14.8 %)
|
|
Lymph node
|
|
9 (16.0 %)
|
9 (10.2 %)
|
|
Gynecologic
|
1 (3.2 %)
|
4 (7.0 %)
|
5 (5.7 %)
|
|
Soft tissue
|
1 (3.2 %)
|
2 (3.5 %)
|
3 (3.4 %)
|
|
Ear, Nose, Throat
|
-
|
2 (3.5 %)
|
2 (2.3 %)
|
|
Urologic
|
-
|
3 (5.9 %)
|
3 (3.4 %)
|
|
Othersa
|
2 (6.4 %)
|
3 (5.9 %)
|
5 (5.7 %)
|
|
Stage
|
|
|
|
0.091
|
|
Locally
advanced
|
24 (77.4 %)
|
45 (79.0 %)
|
69 (78.4 %)
|
|
Metastatic
|
7 (22.6 %)
|
12 (21.0 %)
|
19 (21.6)
|
a: skin, oral cavity, lung, eye.
Characteristics
of CRLC
Among the 51 CRLC episodes, grade 2 complications predominated,
representing 100% of phlebitis and 59.1% of extravasations. Most complications
occurred during the first four cycles. Evolution was favorable within 10 days
in 100% of phlebitis cases (Table 4).
Table 3. Therapeutic characteristics according
to the occurrence of CRCL
-
Grade 2
extravasation: local care after blister rupture and, in some cases, ice
application were provided, leading to complete recovery (Figure 2).

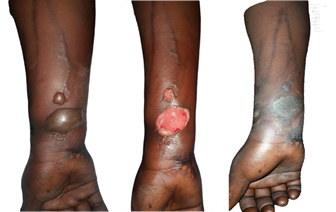
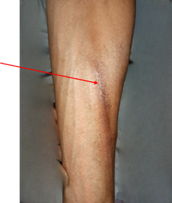
Figure 2. Extravasation secondary to chemotherapy under the EC protocol, grade 2
according to CTCAE v5.0. (A) presence of blisters on the inner aspect of the
lower third of the right forearm, associated with serpiginous hyperpigmentation
along the venous pathways. (B) blister rupture; (C) healing with residual
hyperpigmentation after 13 days of local care.
-
Severe
complications or delayed consultation: some extravasations
progressed to extensive lesions required surgical excision in 9.1% of cases
(Figure 3). These interventions did not result in any permanent functional
sequelae but led to temporary interruption in chemotherapy until clinical
improvement was achieved. Residual hyperpigmented scars were observed, without
impact on mobility or treatment resumption.
No specific preventive measures were implemented to limit the
occurrence of phlebitis or extravasation.


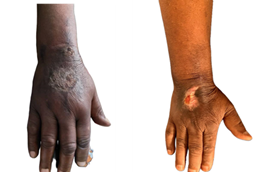
Figure 3. Extravasation following AC
chemotherapy. (A) Necrotic plaque on the dorsum of the right hand observed on
day 17 of the cycle, corresponding to grade 3 according to CTCAE v5.0. (B) Scar
appearance three months after surgical excision of the lesions, which led to a
temporary interruption of treatment.
Discussion
CRCL, such as
phlebitis and extravasation, represent frequent clinical challenges that remain
underreported, especially in resource-limited settings. These complications not
only impair patient quality of life but also threaten treatment continuity. In
our study conducted at Donka University Hospital, Guinea, we observed a notable
incidence of phlebitis and extravasation closely linked to contextual,
structural, and organizational factors specific to low-resource environments.
In our study,
35.2% of patients experienced at least one CRLC episode, corresponding to 26.4%
of the 193 chemotherapy cycles administered. Phlebitis was the most common
complication (15.0% of cycles), followed by extravasation (11.4%). These rates
exceed those reported in some well-resourced settings but remain lower than
incidences documented in other low-resource contexts where phlebitis prevalence
may exceed 30% (14,15). Conversely,
in high-resource countries, extravasation incidence remains very low (<1%),
despite peripheral venous access being a known risk factor for vesicant agents (16).
A major
determinant of CRLC in our study was the near-exclusive use of peripheral
venous access. Unlike well-equipped centers where central venous devices
(implantable ports, central catheters) are commonly used, these devices are
rarely available in our context due to economic and supply constraints. This
results in repeated punctures at a limited number of sites, creating a
favorable environment for phlebitis and extravasation. This pattern aligns with
findings from other studies on chemotherapy local complications in sub-Saharan
Africa and other low-resource settings (14,17,18).
Our findings
confirm that CRLC are influenced by structural, organizational, and clinical
factors specific to our environment. The absence of standardized management
protocols, shortages of specific antidotes, and insufficient healthcare staff
training contribute to both the occurrence and suboptimal management of these
events. Additionally, the high frequency of locally advanced cancer stages
treated with aggressive chemotherapy protocols increases the risk of local
toxicities, especially with vesicant agents like anthracyclines and docetaxel.
These factors combine with individual risk factors previously described in the
literature, including venous fragility, obesity, comorbidities, and lymphedema (19–21).
Therapeutically,
anthracycline-based protocols and docetaxel monotherapy were the most
frequently used, both classes known for local toxicity. Anthracyclines, being
vesicants, pose a significant risk of tissue necrosis in case of extravasation,
requiring timely administration of specific antidotes such as dexrazoxane
within six hours, alongside appropriate physical measures (cold or heat,
depending on the agent) (8, 9, 19, 22). Docetaxel, an irritant agent, can cause
local reactions like phlebitis or skin eruptions, often occurring shortly after
infusion (22, 23).
Most CRCL
occurred during the first four cycles, consistent with other studies such as
Roberts et al. (12), who reported
27% of patients developing severe phlebitis after three anthracycline cycles.
This phenomenon may be explained by vein fragility during initial infusions,
absence of progressive adaptation, and concentration of infusions on a limited
number of venous sites. This underscores the importance of vigilant monitoring
and preventive strategies early in treatment.
Management
primarily involved symptomatic measures (infusion cessation, site change, local
care). Severe cases (9.1%) required surgical excision, leading to temporary
chemotherapy interruption but no permanent functional sequelae. These findings
highlight the critical need for early and adequate management to prevent
complications from worsening.
However, this
study has some limitations. The sample size and the relatively short duration
of data collection (3 months) may limit the scope of the results and their
generalization. Furthermore, the lack of immediate paraclinical diagnostic
tools, such as emergency Doppler ultrasound or thermography, may have led to an
underestimation of lesions, particularly for extravasation. Additionally, the
fact that this study is monocentric and primarily descriptive also prevents the
establishment of firm causal links between the studied factors and the observed
complications.
Despite these
limitations, the results of this study open several avenues for improving the
management of chemotherapy-related local complications in similar contexts. It
is crucial to strengthen the continuous training of medical staff and establish
standardized protocols for the prevention and treatment of phlebitis and
extravasation. The introduction of central venous access devices and the
availability of specific antidotes are also priorities for reducing the
incidence of these complications. Furthermore, future studies should explore
the impact of using central venous access devices on the reduction of local
complications and implement more precise diagnostic approaches to improve early
detection. Finally, research could focus on the evaluation of early management
of complications in a resource-limited setting, in order to better prevent
long-term sequelae.
Conclusion
Our study highlights a high frequency of CRLC, mainly phlebitis and
extravasation, in a resource-limited setting. These events, often early and
moderately severe, are favored by the near-exclusive use of peripheral venous
access and remain underreported, partly due to structural and organizational
constraints hindering their prevention and optimal management.
These results underscore the urgent need to improve the safety of
oncological care through realistic and context-appropriate measures: enhancing
staff competencies, educating patients, and improving access to secure venous
devices and specific treatments. Such interventions would contribute to
preserving patient quality of life, preventing treatment interruptions, and
ensuring safer, more equitable care.
Despite its limitations, this study provides original prospective
data from a Francophone African setting that is underrepresented in the
literature. It calls for multicentric research and healthcare system
strengthening strategies to reduce the impact of these complications on the
treatment journey of cancer patients in Guinea and similar contexts.
Author contribution
BT conceived the study. AMC and IKC collected and
analyzed the data. IKC and AMC drafted and revised the
manuscript. BT and KC supervised the study and validated the
scientific content. All authors reviewed and approved the final manuscript.
Conflicts of interest
There are no conflicts of interest.
Funding
There is no funding.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN
Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries.
CA Cancer J Clin. 2021;71(3):209‑49.
2. Ferlay J, Ervik M, Lam
F, Laversanne M, Colombet M, Mery L, et al. Global Cancer Observatory: Cancer
Today. International Agency for Research on Cancer. 2024 (cited 2025 Aug 9);
Available from: https://gco.iarc.who.int/today/en
3. National Cancer Institute. Types of Cancer Treatment
(Internet). Bethesda (MD): National Cancer Institute; 2017 (cited 2025 Aug 9).
Available from: https://www.cancer.gov/about-cancer/treatment/types
4. National Cancer Institute. Chemotherapy to TreatCancer
(Internet). Bethesda (MD): National Cancer Institute; 2015 (cited 2025 Aug 9).
Available from:
https://www.cancer.gov/about-cancer/treatment/types/chemotherapy
5. de Wit M, Ortner
P, Lipp HP, Sehouli J, Untch M, Ruhnke M, et al. Management of cytotoxic
extravasation - ASORS expert opinion for diagnosis, prevention and treatment.
Onkologie. 2013;36(3):127‑35.
6. Webster J, McGrail M,
Marsh N, Wallis MC, Ray-Barruel G, Rickard CM. Postinfusion Phlebitis:
Incidence and Risk Factors. Nurs Res Pract. 2015;2015:691934.
7. Matloob R, Alikhasi A,
Shirkhoda M, Najafi M. Breast Necrosis after Chemotherapy for Recurrent Ovarian
Cancer: Report of a Case and Review of the Literature. Breast J.
2015;21(4):418‑22.
8. Ener RA, Meglathery SB,
Styler M. Extravasation of systemic hemato-oncological therapies. Annals of
Oncology. 2004;15(6):858‑62.
9. Pérez Fidalgo JA,
García Fabregat L, Cervantes A, Margulies A, Vidall C, Roila F. Management of
chemotherapy extravasation: ESMO–EONS Clinical Practice Guidelines. Annals of
Oncology. 2012;23:vii167‑73.
10. Banjarnahor S. The
Correlation between Chemotherapy (Vesikan) and Extravasation in Cancer Patients
in Teguh Pure Hospital in 2018. Science Midwifery. 2018;7(1):1‑10.
11. Harris V, Hughes M,
Roberts R, Dolan G, Williams EM. The Development and Testing of a
Chemotherapy-Induced Phlebitis Severity (CIPS) Scale for Patients Receiving
Anthracycline Chemotherapy for Breast Cancer. J Clin Med. 2020;9(3):701.
12. Roberts R, Borley A,
Hanna L, Dolan G, Ganesh S, Williams EM. Identifying Risk Factors for
Anthracycline Chemotherapy-induced Phlebitis in Women with Breast Cancer: An
Observational Study. Clinical Oncology. 2021;33(4):230‑40.
13. Ramashia PN, Nkosi PB,
Mbonane TP. Barriers to Radiotherapy Access in Sub-Saharan Africa for Patients
with Cancer: A Systematic Review. IJERPH. 2024;21(12):1597.
14. Sudhakar S, Jacob A,
Hegde NN, Chaudhary RK, Mateti UV, Shetty V. Evaluation of chemotherapy
infusion and phlebitis among cancer patients: A prospective observational
single-center study. J Oncol Pharm Pract. 2023;29(8):1944‑50.
15. Somayaji S, Reddy SS,
Krishna Murthy M, Maka VV. 432P - Chemotherapy induced extravasation: Incidence
and possible predictors. Annals of Oncology. 2019;30:ix143‑4.
16. Jackson-Rose J, Del
Monte J, Groman A, Dial LS, Atwell L, Graham J, et al. Chemotherapy
Extravasation: Establishing a National Benchmark for Incidence Among Cancer
Centers. Clin J Oncol Nurs. 2017;21(4):438‑45.
17. Abd El-Salaheen M, Ahmed
B, Mahmoud A. Correlates to extravasation among patient receiving chemotherapy
at a university hospital. Egypt Nurs J. 2018;15(1):71.
18. Lulie M, Tadesse A,
Tsegaye T, Yesuf T, Silamsaw M. Incidence of peripheral intravenous catheter
phlebitis and its associated factors among patients admitted to University of
Gondar hospital, Northwest Ethiopia: a prospective, observational study. Thrombosis
J. 2021;19(1):48.
19. Schulmeister L.
Extravasation Management: Clinical Update. Seminars in Oncology Nursing.
2011;27(1):82‑90.
20. Lv L, Zhang J. The
incidence and risk of infusion phlebitis with peripheral intravenous catheters:
A meta-analysis. J Vasc Access. 2020;21(3):342‑9.
21. Mandal A, Raghu K. Study
on incidence of phlebitis following the use of pherpheral intravenous catheter.
J Family Med Prim Care. 2019;8(9):2827‑31.
22. Zhu LL, Wang Y hong,
Zhou Q. Progress in Research on the Mechanisms and Interventions of Phlebitis
from the Perspective of Vascular Endothelial Cell and Signaling Pathway. J Inflamm Res. 2023;16:6469‑81.
23. Raley J, Geisler JP, Buekers TE, Sorosky JI.
Docetaxel Extravasation Causing Significant Delayed Tissue Injury. Gynecologic
Oncology. 2000;78(2):259‑60.