Introduction
Thymic
malignancies are relatively slow-growing tumours with thymoma being
potentially malignant and thymic carcinoma being malignant with
origin being thymic epithelial cell (1,2). The histopathological classification and staging
classification are based on World Health Organization (WHO) 2021 classification
and the modified Masoka staging system (3,4).
Patients presenting with thymic
malignancies are uncommon, even in a tertiary centre. There are very few
studies with large cohorts, especially in the Indian setting, with most being
retrospective studies with small numbers of cases and this explains the unmet
needs in standardisation of treatment approaches. The data on exact incidence
in Indian population is lacking due to scarce widespread epidemiological data.
Surgical resection remains the cornerstone
of treatment for patients with resectable thymic malignancies. For those with
unresectable tumors, neoadjuvant chemotherapy or definitive radiotherapy are
commonly employed therapeutic strategies. In patients with advanced or
metastatic disease, systemic palliative therapy is the standard approach,
although data on available combination regimens are
limited.
This retrospective study outlines our clinical experience in the
management of thymic malignancies in patients treated between January 2022 and
May 2025.
The purpose of this study is to
share experience related to epidemiology, clinical presentation, management,
and treatment outcomes, and prognostic relations to different clinical
parameters, with special emphasis on the experience of systemic combination
therapy in these patients. The objective of the study is to evaluate and report
outcomes associated with combination chemotherapy in thymoma based on our
clinical experience.
Materials and methods
A retrospective study of patients diagnosed
between January 2022 to May 2025 with thymic malignancies was conducted from a regional cancer centre in the
southern part of India. All the patients diagnosed with thymic malignancies,
irrespective of stage or criteria were included in
the study. Data were collected for epidemiological, clinical,
histopathological, staging, and treatment outcome from patient files or
electronic data. SPSS program version 23.0 for Windows was
used for data analysis. To examine the relationship between ordinal
variable, Chi-square test was used.
Most patients were
evaluated with contrast-enhanced computed tomography scan (CECT)
(Thorax/abdomen/pelvis), few with positron emission tomography-computed
tomography (PET/CT) imaging. Patients were staged
based on the modified Masoka staging system (6). Image-guided biopsy was done,
and all specimens were reviewed by an
oncopathologist and reported based on 2021 WHO classification of tumours of
thymus (4). Patients with symptoms not related to primary or metastasis were evaluated for paraneoplastic syndrome.
All resectable patients after informed
consent underwent upfront resection. Patient with potentially resectable tumour
received neoadjuvant chemotherapy followed by surgical review. Patient with
high risk factor (margin positive, prior neoadjuvant chemotherapy, masoka stage
II or higher) received adjuvant radiation therapy. Patients believed to be
unresectable despite neoadjuvant chemotherapy or with extensive disease
received palliative chemotherapy. Post definitive management, patients were kept on surveillance with 6-monthly CECT thorax,
while imaging was performed every 3 months in
patients on palliative treatment.
Results
A total of 52
patients were diagnosed with thymic malignancy
(thymoma/thymic carcinoma) during January 2022 to May 2025. Out of 52 patients,
data for analysis were available for 42 patients. Male to female ratio was
1.2:1, with a median age of 49 years (18-67 years), with 26% of patients below
the age of 40 years. 28.5% of the patients had co-morbidities (Table 1).
Most common presentations were ptosis, cough,
breathing difficulty, chest pain, and hoarseness of voice. One patient
presented incidentally. Either prior to referral to a tertiary centre or on
evaluation, 31% of patients had myasthenia gravis, many
of them presented with ptosis and a few had breathing difficulties. For all
patients with myasthenia, the neurologist's opinion was
taken prior to starting oncological management. Most of the patients were treated with pyridostigmine.
Three patients required plasmapheresis, while
azathioprine and mycophenolate mofetil were each given to two patients. One
patient received two doses of rituximab. None of the patients had pure red cell
aplasia (Table 1).
Table 1. Demographic
characteristics and clinical presentation.
|
Patients |
Numbers |
Percentage |
|
Age |
49 (18-61) |
|
|
Sex |
|
|
|
Male |
23 |
54.7% |
|
Female |
19 |
45.2% |
|
Comorbidities |
|
|
|
Diabetes |
5 |
11.9% |
|
Hypertension |
9 |
21.4% |
|
Ischemic Heart Disease |
3 |
7.1% |
|
Presentation |
|
|
|
Cough |
10 |
23.8% |
|
Ptosis |
10 |
23.8% |
|
Dyspnea |
9 |
21.4% |
|
Chest Pain |
6 |
14.2% |
|
Generalised Weakness |
5 |
11.9% |
|
Hoarseness |
5 |
11.9% |
|
Shoulder Pain |
1 |
2.38% |
|
Incidental |
1 |
2.38% |
|
Myasthenia Gravis |
13 |
30.9% |
On evaluation, 36 patients had thymoma,
while 6 patients had thymic carcinoma (14.3%). Among patients with thymoma, B2 &B3
were common, making up of 33.3% and 21.4% of all 42 patients, respectively.
Type A was 16.7% and type B1 was 11.9%. One patient
was diagnosed with the spindle cell type of
thymoma. Among these patients, 23.8%, 21.4%, 23.8%, and 31.0% had stage I, II,
III, and IV, respectively (Table 2). Among patients
with stage IV disease, 8 out of 13 had evidence of extra-thoracic metastasis.
Five out of six thymic carcinoma cases had stage IV disease. The most common sites of metastasis were pleura and pleural effusion,
lung, non-regional lymph node, adrenal, and liver. Patient with type A/B1
presented with stage I/II, while thymic carcinoma was more common in patients
with stage IV (Figure 1).
Table
2. Clinical and pathological characteristics of the study population.
|
Disease status |
No. Of patients |
Percentage |
|
Type of
malignancy |
|
|
|
Thymectomy
carcinoma |
6 |
14.3% |
|
Thymoma |
36 |
85.7% |
|
A |
7 |
16.7% |
|
B1 |
5 |
11.9% |
|
B2 |
14 |
33.3% |
|
B3 |
9 |
21.4% |
|
Metaplastic |
1 |
2.4% |
|
Staging |
|
|
|
I |
10 |
23.8% |
|
II |
9 |
21.4% |
|
IIIa |
4 |
9.5% |
|
IIIb |
6 |
14.3% |
|
IVa |
6 |
14.3% |
|
IVb |
7 |
16.7% |
|
Sites
of metastasis |
|
|
|
Pleura
and pleural effusion |
9 |
21.4% |
|
Lung |
4 |
9.5% |
|
Non
regional Lymph nodes |
2 |
4.8% |
|
adrenal |
1 |
2.4% |
|
Liver |
1 |
2.4% |
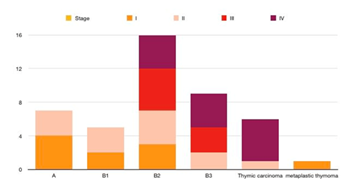
Figure 1. Stage-wise distribution of patients in
different histotypes.
Out of the total
patients, 23 patients (stage I and II and few stage III were
believed resectable upfront and underwent surgery. Most of the patients
underwent video-assisted thoracic surgery (VATS). Twelve patients (stage III
and IVa) were started on neoadjuvant chemotherapy.
Following neoadjuvant chemotherapy, 4 patients achieved a partial response, 3
patients had stable disease, and 5 patients showed disease progression. Out of 12 patients, 5 patients underwent surgery,
but 1 patient had residual disease post-surgery and was
treated with definitive RT. Patient with high-risk factors received
adjuvant RT. A total of 19 patients received chemotherapy either as neoadjuvant
or a palliative setting (Table 3).
Table 3. Summary of treatment
history.
|
Treatment |
No of patients |
Percentage |
|
Upfront
surgery |
23 |
54.7% |
|
Neoadjuvant
systemic therapy |
12 |
28.5% |
|
Post
neoadjuvant R0 Resection |
4 |
9.5% |
|
Adjuvant
radiation |
7 |
16.7% |
|
Unresectable
patients |
15 |
35.7% |
|
Definitive
RT (residual post surgery) |
1 |
2.4% |
|
Chemotherapy |
19 |
45.2% |
|
Type of
chemotherapy |
|
|
|
ADOC |
9 |
21.4% |
|
CAP |
6 |
14.3% |
|
paclitaxel-carboplatin |
4 |
9.5% |
The most used
chemotherapy regimen in thymoma was combination adriamycin, cisplatin,
vincristine, and cyclophosphamide (ADOC) in 9 patients and combination
cyclophosphamide, adriamycin and cisplatin (CAP) in 6 patients.
Paclitaxel-carboplatin was used in patients with
thymic carcinoma (5 patients). Disease control rate was
seen in 68.4 % of the patients with an overall response rate (ORR) of
52.6%. Two of the patients achieved a complete response. Response rates with
ADOC, CAP and Palitaxel-carboplatin were 55.5%, 50% and 40% (p value-0.31),
respectively. Out of 9 patients who progressed, 5 received second line systemic
therapy – single agent pemetrexed (3) and two received combination etoposide,
ifosfamide, and cisplatin (VIP). All patients who received ADOC and CAP
received growth factors. Neutropenia was seen in
42.1% of the patients, with Grade ¾ seen in 15.7% of the patients (3 out of
19). However, it did not lead to discontinuation of systemic therapy.
Adriamycin dose was reduced in 2 patients. Six
patients had peripheral neuropathy (31.5%), with grade 3 peripheral neuropathy
seen in one patient (5.2%). Grade 2 Diarrhoea was seen
in 3 patients (15.7%). Nausea/vomiting was seen in
6 patients (31.5%). One patient developed grade 2 hyponatremia. There was no treatment-related
death seen with systemic therapy (Table 4).
Table 4. Toxicity profile.
|
Toxicity |
All grade |
Grade 3/4 |
|
Overall toxicity |
68.4% |
26.3% |
|
Hematological toxicity |
|
|
|
Neutropenia |
42.1% |
15.7% |
|
Febrile neutropenia |
10.5% |
0% |
|
Anemia |
52.6% |
10.5% |
|
Thrombocytopenia |
15.7% |
0% |
|
Non hematological toxicity |
|
|
|
Nausea /vomiting |
31.5% |
0% |
|
Diarrhoea |
15.7% |
5.26% |
|
Peripheral neuropathy |
31.5% |
5.2% |
|
Hyponatremia |
5.26% |
0% |
|
Mucositis |
21.0% |
5.26% |
|
Increased creatinine |
10.5% |
0% |
After a median
follow of 22 months (2-39 months), in patients who underwent surgery,
recurrences were very less (15.7%). Patient with unresectable disease (15)
progression was seen in 9 patients with a 2-year progression-free
survival (PFS) rate of 58%. 2-yr PFS in patients with stage III was 72% while
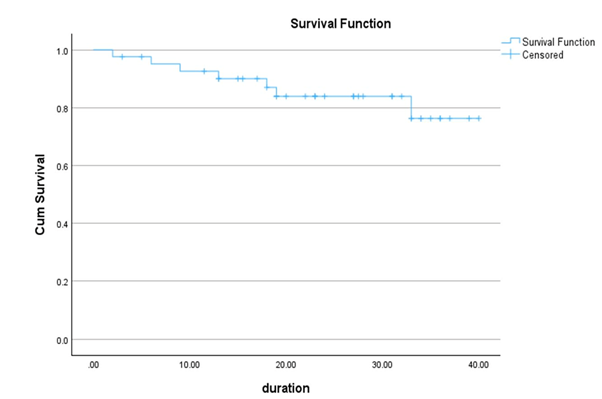
in stage IV it was 50%. After a median follow up 22 months (2-39 months), 6
patients died with a 2-year survival rate of 84%. 2-year survival rates in
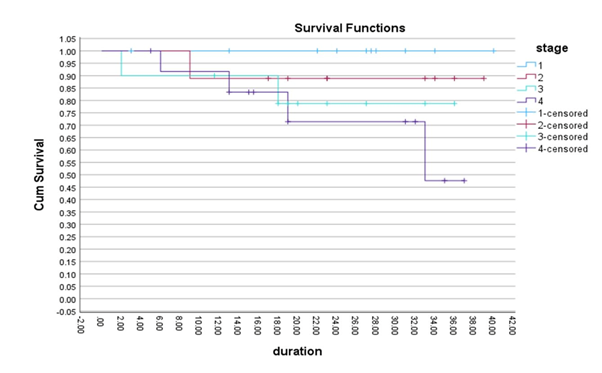
stage I, II, III and IV were 100%, 89%, 79% and 71% respectively (p value
0.03). Overall Survival (OS) rates were lower in thymic carcinoma and patients
with Extra-thoracic disease (2-year OS 50% and 50% respectively) (Figures 2 and
3).