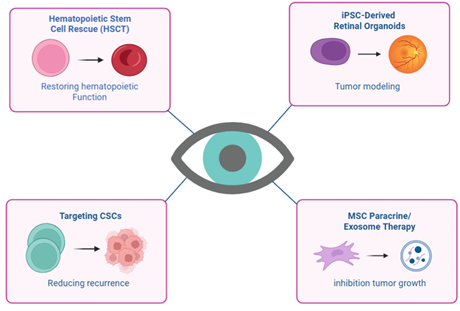
Introduction
RB represents the most prevalent primary intraocular
malignancy occurring in pediatric populations and is predominantly attributed
to biallelic mutations in the RB1 gene (1), and occurs with an incidence of
approximately 15,000 to 20,000 live births (2). Traditional interventions, including systemic chemotherapy,
intra-arterial chemotherapy, radiotherapy, and enucleation, are effective in
achieving tumor control; however, long-term adverse effects and
vision impairment continue to pose significant challenges. Consequently, there
is a pressing need for innovative therapeutic approaches, with stem cell-based
therapies emerging as promising modalities for both treatment and disease
modeling (1). Although these treatments have significantly improved patient outcomes,
they are associated with long-term complications, including secondary
malignancies, growth disturbances, and severe visual impairment (3). Furthermore, resistance to standard chemotherapy is increasingly
acknowledged, in part attributable to the presence of CSCs
within RB tumors, which contribute to tumor recurrence and metastasis (4). Stem cells can be utilized as adjunctive therapy, exemplified by
hematopoietic stem cell transplantation (HSCT), which serves to restore bone
marrow function following high-dose chemotherapy (5). iPSCs and retinal organoids serve as disease models for investigating
the pathogenesis of RB and for
evaluating the efficacy of novel therapeutic agents, as possible therapeutic
targets, concentrating on CSC populations within tumors and in the context of
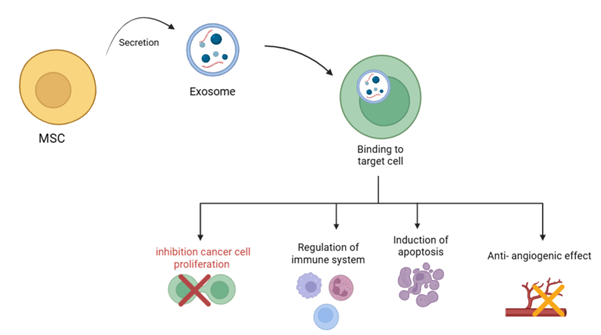
cellular delivery systems, where MSCs or their extracellular vesicles (EVs) are
employed to transport therapeutic molecules directly to tumor locations. These
varied applications underscore the significance of stem cell research (5-7).
Different Types
of RB
The RB1 gene, situated at the chromosomal locus 13q14, encodes the RB
protein, which plays a critical role in controlling the G1/S transition of the
cell cycle through the suppression of E2F transcription factors (8). The inactivation of both functional RB1 alleles results in the
deregulation of retinal cell proliferation, ultimately contributing to
tumorigenesis (9). RB manifests in both heritable or germline and non-heritable or
sporadic forms. In the heritable variant, which follows an autosomal dominant
inheritance pattern, approximately 40% of cases involve children inheriting or
acquiring a germline mutation in one allele of the RB1 tumor suppressor gene.
This mutation is found in all somatic cells, including germ cells, making it
heritable to subsequent generations (10). The two-hit hypothesis posits that tumor development necessitates a
second somatic mutation, resulting in the biallelic inactivation of the RB1
gene within retinal cells (11). Individuals with hereditary RB frequently exhibit bilateral and
multifocal tumors, and there exists a 50% probability that they will transmit
the mutant allele to their offspring (12). Significantly, they are also inclined to develop secondary
malignancies, including osteosarcoma and soft tissue sarcomas (13). In the sporadic cases, in 60% of them, both RB1 mutations manifest
somatically within a single retinal cell. This indicates the absence of a
germline mutation, rendering the condition non-heritable (14). These patients generally exhibit unilateral, solitary tumors and do not
possess an elevated risk of transmitting the disease to their progeny (10).
HPCS Rescue
after High-Dose Chemotherapy
High-dose chemotherapy has been utilized in advanced and refractory RB
cases to address resistance to conventional treatment protocols and to
eliminate minimal residual disease (15, 16). Nevertheless, this intensive chemotherapy regimen is highly
myeloablative, leading to severe and potentially life-threatening suppression
of bone marrow function (17, 18). To mitigate this toxicity, HSCT, primarily utilizing autologous
peripheral blood stem cells, has been established as a rescue approach aimed at
reinstating hematopoietic function (19). Investigations in clinical settings have revealed that the use of
autologous post high-dose chemotherapy leads to enhanced survival rates in
patients diagnosed with extraocular or metastatic RB, concurrently upholding a
tolerable safety profile (20). However, this method entails significant risks, such as infection,
graft failure, and mortality associated with transplantation (21). Therefore, HSCT is generally indicated for pediatric patients
presenting with relapsed or advanced stages of the disease, rather than for
those exhibiting localized intraocular RB (22, 23).Current research efforts are focused on refining patient selection
criteria, optimizing conditioning protocols, and reducing long-term
complications associated with HSCT. Furthermore, the integration of HSCT with
novel therapeutic approaches, including targeted molecular agents and
immunotherapies, holds promise for augmenting its efficacy in the future
management of RB (24-26). New methods are being investigated to lower transplant-related
morbidity. Studies are assessing reduced intensity conditioning strategies
aimed at minimizing toxicities while ensuring anti-tumor efficacy, particularly
in very young pediatric patients (27). Moreover, the integration of
HSCT with novel therapeutic approaches, including immune checkpoint
inhibitors and targeted agents, has the potential to enhance long-term clinical
outcomes by effectively targeting both the predominant tumor cell population
and chemoresistant cancer stem-like cells (28, 29).
Stem Cells for
Disease Modeling (iPSCs and Retinal Organoids)
iPSCs derived from patients have significantly advanced the investigation
of human diseases, including RB (30, 31). These iPSCs are produced by reprogramming somatic cells, such as
fibroblasts, into a pluripotent state, thereby conferring the capacity to
differentiate into diverse cell types, including retinal cells (32, 33). This methodology facilitates the generation of retinal organoids as
three-dimensional constructs that faithfully replicate the cellular
organization and developmental dynamics of the human retina in vitro (34-36). iPSCs derived from patients harboring RB1 mutations enable the direct
investigation of tumor initiation within a genetic context that closely
replicates the patient’s disease profile, thereby bridging the divide between
experimental models and clinical scenarios (37, 38). This approach is particularly advantageous given the inherent
challenges of studying RB in vivo, which stem from its rarity, heterogeneity,
and the ethical constraints associated with pediatric tumor research. The
resultant retinal organoids comprise proliferative retinal progenitor cells
that recapitulate the hyperplastic lesions characteristic of RB patients (39-41). Such models are instrumental for elucidating the molecular mechanisms
underpinning tumorigenesis, including the regulatory pathways governing cell
proliferation, apoptosis, and differentiation. Furthermore, retinal organoids
facilitate the exploration of tumor microenvironment interactions. Co-culture
systems integrating retinal organoids with immune or stromal cells permit the
examination of the contributions of non-tumor cell populations to RB
progression, immune evasion, and therapeutic resistance (42, 43). Another significant application lies in high-throughput drug screening,
where organoid-based platforms enable the assessment of chemotherapeutic
agents, targeted inhibitors, and gene silencing techniques under
physiologically relevant conditions (44, 45). This methodology not only forecasts therapeutic efficacy but also aids
in identifying patient-specific drug sensitivities, thereby advancing the
prospects of precision oncology in RB (46). Additionally, advancements in bioengineering are propelling the field
towards the development of vascularized and innervated retinal organoids, which
can more accurately replicate the metabolic demands and stress responses of
tumors. These next-generation models may yield insights into the mechanisms of
hypoxia-induced resistance and angiogenesis in RB (47). iPSCs and retinal organoids together constitute a progressively
advancing set of methodologies that surpass traditional modeling approaches.
These tools are increasingly essential for elucidating the biology of RB,
investigating tumor host interactions, and facilitating the development of
personalized therapeutic interventions (48).
Targeting RB
CSCs
One of the new paradigms in RB research is the acknowledgment of CSCs as
a vital factor in tumor initiation, progression, therapeutic resistance, and
recurrence. CSCs found in RB tumors demonstrate the ability for self-renewal,
multipotency, and tumorigenicity, resembling normal stem cells, albeit with
dysregulated pathways that govern proliferation and differentiation (49). Crucially, these cells are believed to persist following conventional
treatments, including chemotherapy and radiotherapy, thereby serving as a
reservoir for disease recurrence (50). Several molecular signaling pathways, notably Notch, Hedgehog (HH),
Wingless-related Integration Site / β-catenin (Wnt/β-catenin), and
Phosphoinositide 3-kinase/ Protein Kinase B/ mammalian Target Of Rapamycin (PI3K/Akt/mTOR), are frequently involved in
sustaining the CSC phenotype in RB. Dysregulated activation of these pathways
facilitates survival signaling, epithelial to mesenchymal transition (EMT), and
metabolic reprogramming, enabling CSCs to adapt to hypoxic or nutrient-deprived
microenvironments. Accordingly, therapeutic interventions targeting these
pathways, such as Gamma Secretase Inhibitors (GSIs) for Notch, Smoothened (SMO)
antagonists for HH, and Wnt pathway inhibitors, have garnered considerable
interest in preclinical studies (51, 52). Additionally, immunotherapeutic strategies specifically directed
against CSCs represent a promising avenue. For example, CSCs in RB frequently
overexpress markers including ATP-binding cassette subfamily G member 2
(ABCG2), Cluster of Differentiation 44 (CD44), and Aldehyde Dehydrogenase
Family 1 (ALDH1), which may serve as viable therapeutic targets. Approaches
employing monoclonal antibodies, chimeric antigen receptor T (CAR-T) cells, or
antibody drug conjugates designed to eradicate CSCs selectively have
demonstrated preclinical efficacy in other solid tumors and hold potential for
adaptation in RB treatment (53, 54). Furthermore, the inhibition of drug efflux transporters such as ABCG2
may increase the sensitivity of CSCs to chemotherapy, potentially addressing
the issue of multidrug resistance (55). In addition, epigenetic regulation has emerged as a critical factor
influencing CSC survival in RB. Aberrant patterns of histone modifications and
deoxyribonucleic acid (DNA) methylation contribute to the maintenance of
stemness-associated gene expression. Consequently, epigenetic inhibitors,
including histone deacetylase (HDAC) inhibitors and DNA methyltransferase
inhibitors, are under investigation for their capacity to induce
differentiation in CSCs and enhance their responsiveness to therapeutic interventions
(56, 57). Collectively, targeting CSCs in RB signifies a paradigm shift from
traditional treatments that predominantly focus on eliminating the rapidly
proliferating tumor mass. By specifically eradicating the rare,
therapy-resistant CSC population, it may be possible to achieve sustained
remission and reduce recurrence rates. The integration of CSC targeted
strategies with current treatment modalities, such as intra-arterial
chemotherapy and focal therapies, holds promise for improving long-term
therapeutic outcomes in patients with RB (58).
Targeting CSCS
Signaling in RB
CSCs depend on developmental signaling pathways to maintain their
self-renewal capabilities and ensure their survival. Investigating and
therapeutically targeting these pathways presents a promising approach for the
eradication of CSCs and the enhancement of treatment efficacy (59) (Table 1). The Wnt/β-catenin signaling
pathway plays a vital role in sustaining CSC self-renewal. Abnormal activation
of Wnt signaling has been observed in RB, which fosters cell proliferation and
confers resistance to standard therapies. The pharmacological blockade of Wnt
signaling through the use of small molecules or inhibitors can inhibit the
nuclear translocation of β-catenin, diminish the expression of CSC
markers, and make tumor cells more susceptible to chemotherapy (Figure 1).
Additionally, targeting Wnt signaling may reduce metastatic capabilities by
disrupting EMT processes (60).
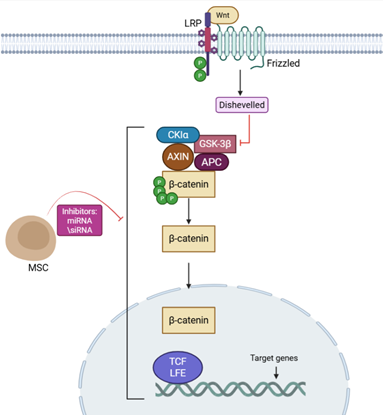
Figure 1. Activation of the β-catenin pathway
in RB leads to β-catenin accumulation and transcription of target genes
that promote tumor growth and stem cell maintenance. MSCs, through secreted
factors and messenger RNAs(mRNAs), can inhibit this pathway, suppressing
β-catenin activity and reducing tumor proliferation.
Notch signaling is key to
controlling cell development and supporting stem cell maintenance. In RB,
overactive Notch pathways promote blood vessel growth and help CSCs survive (60). Lab studies show that drugs like GSIs and antibodies blocking Notch
receptors can slow CSC growth and reduce tumor blood vessels, potentially
making standard chemotherapy more effective by overcoming drug resistance.
Similarly, the HH pathway, especially Sonic Hedgehog (SHH), drives tumor growth
and CSC survival by interacting with Patched receptors to activate SMO and GLI
proteins, which control genes for cell growth and stem-like traits (61). Drugs like vismodegib and sonidegib, which block SMO, have been shown
to shrink CSC populations and slow RB tumor growth in lab models. The
PI3K/AKT/mTOR pathway also plays a major role in CSC survival, growth, and
metabolism, contributing to drug resistance in RB. Using targeted drugs to
block PI3K, AKT, or mTOR can trigger CSC death, reduce their ability to
self-renew, and make tumors more responsive to standard treatments (62, 63). Combining therapies that target PI3K/AKT/mTOR with those hitting Wnt or
Notch pathways may create stronger anti-CSC effects (Figure 2).
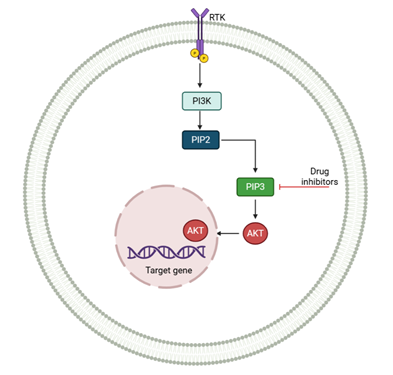
Figure 2. PI3K/AKT signaling pathway modulated by
MSC-derived exosomes in RB. Activation of RTK leads to PI3K-mediated
phosphorylation of PIP2 to PIP3, triggering AKT activation and subsequent
transcription of genes promoting survival, proliferation, and drug resistance.
Targeted inhibitors can block this pathway to suppress tumor progression.
Focusing on the molecular pathways of CSCs in RB offers a hopeful
strategy to tackle tumor regrowth and drug resistance. Combining CSC-specific
treatments with traditional chemotherapy or radiation could target both the
main tumor cells and the resistant CSC group, improving long-term outcomes and
lowering the chance of the cancer returning (64, 65). Further lab and human studies are needed to refine drugs that target
these pathways and to develop effective treatment combinations.
Limitations and
Challenges
Stem cell therapies hold great potential for treating RB, but significant
hurdles must be addressed before they can be widely used in clinics. MSCs have
dual roles: they can deliver anti-cancer agents or modify the tumor
environment, but they may also release factors that unintentionally promote
tumor growth, blood vessel formation, or spread (85). To ensure MSCs only provide benefits, careful analysis and modification
of these cells are crucial. Long-term safety remains a major concern, with
risks including the potential for transplanted cells to form tumors, trigger
immune reactions, or cause unintended effects, especially in children with RB.
Genetically altered stem cells require rigorous testing to avoid risks like
gene mutations or activation of cancer-causing genes. Despite their ability to
target tumors, getting stem cells to reach eye tumors consistently is difficult
due to barriers like the blood-retina barrier, fluid dynamics in the eye, and
immune defenses. Optimizing delivery methods, cell doses, and timing is key to
improving treatment success (86). RB’s genetic and cellular diversity, including resistant CSCs,
complicates stem cell treatments and highlights the need for therapies tailored
to each patient’s tumor profile (2, 87). Producing and testing stem cells consistently, including their
exosomes, is vital for clinical use. Factors like growth conditions, cell
passage, and donor differences can affect treatment outcomes, requiring strict
protocols to meet regulatory standards (88). Most stem cell research for RB is still in lab or animal studies, with
few human trials. While these models provide useful insights, clinical studies
are needed to evaluate long-term effects, ideal dosing, and combinations with
standard treatments (89). In summary, stem cell therapies offer exciting possibilities for RB,
but thorough assessments of safety, tumor-specific effects, delivery
approaches, and compliance with regulations are essential. Addressing these
obstacles will unlock the full potential of stem cells in personalized RB
treatment.
Conclusion
Stem cell technologies are reshaping the landscape of RB research and
treatment. iPSCs and retinal organoids have become essential tools for
uncovering disease mechanisms and testing new drugs, paving the way for
personalized cancer care. The identification of CSCs underscores the need to
target tumor-initiating cells to ensure lasting remission and address drug
resistance. Meanwhile, MSC-derived factors and exosomes provide powerful
carriers for delivering anticancer therapies directly to tumors, reducing harm
to healthy tissues. HSC support remains a key complement to standard
chemotherapy, enabling higher doses while limiting long-term side effects.
Together, these strategies highlight how stem cell advances deepen our
understanding of RB and lay the groundwork for innovative, patient-tailored
treatments. Looking ahead, progress will depend on combining lab-based models
with clinical applications, ensuring safety, and maximizing the combined impact
of cellular and molecular therapies.
Author contribution
EB and AN designed the study, as
well as collected and analyzed the data. MM was responsible for drafting
and revising the manuscript. PMS provided supervision for the study and
ensured the scientific accuracy of its content. All authors critically reviewed
and gave their approval for the final version of the manuscript.
Funding
There is no funding.
Conflicts of interest
There are no conflicts of interest.
References
1. Dimaras
H, Corson TW, Cobrinik D, White A, Zhao J, Munier FL, et al. Retinoblastoma.
Nat Rev Dis Primers. 2015;1:15021.
2. Feng
Y, Feng X, Lv Y. Worldwide Burden of Retinoblastoma from 1990 to 2021.
Ophthalmic Research. 2024;67(1):672-82.
3. Schaiquevich
P, Francis JH, Cancela MB, Carcaboso AM, Chantada GL, Abramson DH. Treatment of
Retinoblastoma: What Is the Latest and What Is the Future. Front Oncol.
2022;12:822330.
4. Bao
B, Ahmad A, Azmi AS, Ali S, Sarkar FH. Overview of cancer stem cells (CSCs) and
mechanisms of their regulation: implications for cancer therapy. Curr Protoc
Pharmacol. 2013;Chapter 14:Unit 14.25.
5. Merrien
M. The role of cannabinoid receptors, G alpha z, and B cell receptor in
lymphoma pathobiology with focus on chemotaxis: Karolinska Institutet (Sweden);
2021.
6. Kangari
P, Salahlou R, Vandghanooni S. Harnessing the Therapeutic Potential of
Mesenchymal Stem Cells in Cancer Treatment. Adv Pharm Bull. 2024;14(3):574-90.
7. Ogilvie
JM, McKillop N, Cale J, Allard T, Rynne J, Smallbone S. Assessing the
Effectiveness of a Specialized, Field-Based Treatment Program for Youth Who
Have Committed Sexual Offenses in an Australian Jurisdiction. Int J Offender
Ther Comp Criminol. 2024;68(15):1540-57.
8. Yun
J, Li Y, Xu CT, Pan BR. Epidemiology and Rb1 gene of retinoblastoma. Int J
Ophthalmol. 2011;4(1):103-9.
9. Flores
M, Goodrich DW. Retinoblastoma Protein Paralogs and Tumor Suppression. Front
Genet. 2022;13:818719.
10. Rushlow
DE, Mol BM, Kennett JY, Yee S, Pajovic S, Thériault BL, et al. Characterisation
of retinoblastomas without RB1 mutations: genomic, gene expression, and
clinical studies. The lancet oncology. 2013;14(4):327-34.
11. Li
YP, Wang YT, Wang W, Zhang X, Shen RJ, Jin K, et al. Second hit impels
oncogenesis of retinoblastoma in patient-induced pluripotent stem cell-derived
retinal organoids: direct evidence for Knudson's theory. PNAS Nexus.
2022;1(4):pgac162.
12. Abu-Amero
KK, Kondkar AA, Almontashiri NAM, Khan AM, Maktabi AMY, Hameed S, AlMesfer S.
Genetics of Retinoblastoma: An Overview and Significance of Genetic Testing in
Clinical Practice. Genes (Basel). 2025;16(9).
13. Jang
YJ, Jeong HK, Kong CB, Song WS, Cho WH, Jeon DG, et al. Secondary hematological
malignancies in patients with sarcoma: A single‑center retrospective
study. Oncol Lett. 2024;27(5):211.
14. Houston
SK, Murray TG, Wolfe SQ, Fernandes CE. Current update on retinoblastoma.
International ophthalmology clinics. 2011;51(1):77-91.
15. Cao
S, Wang Q, Zhu G. From Chemotherapy to Targeted Therapy: Unraveling Resistance
in Acute Myeloid Leukemia Through Genetic and Non-Genetic Insights. Int J Mol
Sci. 2025;26(9).
16. Zhang
Y, Xue C, Cui H, Huang Z. High expression of TAZ indicates a poor prognosis in
retinoblastoma. Diagn Pathol. 2015;10:187.
17. Dunkel
IJ, Chan HS, Jubran R, Chantada GL, Goldman S, Chintagumpala M, et al.
High‐dose chemotherapy with autologous hematopoietic stem cell rescue for
stage 4B retinoblastoma. Pediatric blood & cancer. 2010;55(1):149-52.
18. Liu
Z, Huang P, Law S, Tian H, Leung W, Xu C. Preventive effect of curcumin against
chemotherapy-induced side-effects. Frontiers in pharmacology. 2018;9:1374.
19. Rodriguez-Galindo
C, Orbach DB, VanderVeen D. Retinoblastoma. Pediatric Clinics.
2015;62(1):201-23.
20. Dunkel
IJ, Gardner SL, Garvin JH, Goldman S, Shi W, Finlay JL. High-dose carboplatin,
thiotepa, and etoposide with autologous stem cell rescue for patients with
previously irradiated recurrent medulloblastoma. Neuro-oncology.
2010;12(3):297-303.
21. Timsit
JF, Sonneville R, Kalil AC, Bassetti M, Ferrer R, Jaber S, et al. Diagnostic
and therapeutic approach to infectious diseases in solid organ transplant
recipients. Intensive Care Med. 2019;45(5):573-91.
22. Peras
M, Bilić E, Mareković I. Recent Insights into the Pathogenesis,
Diagnostics, and Treatment of BK Virus Infections in Children After
Hematopoietic Stem Cell Transplantation. Pathogens. 2025;14(3).
23. Clarissa
A, Sutandi N, Fath AA. Stem-Cell Therapy Following High-Dose Chemotherapy in
Advanced Retinoblastoma: A Systematic Review. Asia Pac J Ophthalmol (Phila).
2021;10(4):397-407.
24. Algeri
M, Merli P, Locatelli F, Pagliara D. The Role of Allogeneic Hematopoietic Stem
Cell Transplantation in Pediatric Leukemia. J Clin Med. 2021;10(17).
25. Lee
YJ, Jo DH. Retinal organoids from induced pluripotent stem cells of patients
with inherited retinal diseases: A systematic review. Stem Cell Reviews and
Reports. 2025;21(1):167-97.
26. Rozanska
A, Cerna-Chavez R, Queen R, Collin J, Zerti D, Dorgau B, et al. pRB-depleted
pluripotent stem cell retinal organoids recapitulate cell state transitions of
retinoblastoma development and suggest an important role for pRB in retinal
cell differentiation. Stem Cells Translational Medicine. 2022;11(4):415-33.
27. McNamara
JF, Righi E, Wright H, Hartel GF, Harris PN, Paterson DL. Long-term morbidity
and mortality following bloodstream infection: a systematic literature review.
Journal of Infection. 2018;77(1):1-8.
28. Abramson
DH, Mandelker D, Brannon AR, Berger MF, Robbins M, Dunkel IJ, Francis JH.
Cell-free RB1 DNA not detected in the blood of pseudoretinoblastoma patients.
BMJ Open Ophthalmology. 2022;7(1):e001084.
29. Li
F, Yin Y-K, Zhang J-T, Gong H-P, Hao X-D. Role of circular RNAs in
retinoblastoma. Functional & Integrative Genomics. 2023;23(1):13.
30. Aboul-Soud
MAM, Alzahrani AJ, Mahmoud A. Induced Pluripotent Stem Cells (iPSCs)-Roles in
Regenerative Therapies, Disease Modelling and Drug Screening. Cells.
2021;10(9).
31. Qu
S, Xu R, Yi G, Li Z, Zhang H, Qi S, Huang G. Patient-derived organoids in human
cancer: a platform for fundamental research and precision medicine. Mol Biomed.
2024;5(1):6.
32. Chang
CA, Bhagchandani P, Poyser J, Velasco BJ, Zhao W, Kwon H-S, et al. Curative
islet and hematopoietic cell transplantation in diabetic mice without toxic
bone marrow conditioning. Cell reports. 2022;41(6).
33. Zhang
M, Kim S, Yang HW. Non-canonical pathway for Rb inactivation and external
signaling coordinate cell-cycle entry without CDK4/6 activity. Nature
Communications. 2023;14(1):7847.
34. Symmank
D, Richter FC, Rendeiro AF. Navigating the thymic landscape through
development: from cellular atlas to tissue cartography. Genes Immun.
2024;25(2):102-4.
35. Mohanan
P. Artificial Intelligence and Biological Sciences: CRC Press, Taylor &
Francis Group; 2025.
36. Zhang
XH, Jin ZB. Patient iPSC-derived retinal organoids: Observable retinal diseases
in-a-dish. Histol Histopathol. 2021;36(7):705-10.
37. Sun
Y, Su J, Wang X, Wang J, Guo F, Qiu H, et al. Patient-specific iPSC-derived
cardiomyocytes reveal variable phenotypic severity of Brugada syndrome.
EBioMedicine. 2023;95.
38. Jin
M, Xu R, Wang L, Alam MM, Ma Z, Zhu S, et al. Type-I-interferon signaling
drives microglial dysfunction and senescence in human iPSC models of Down
syndrome and Alzheimer’s disease. Cell Stem Cell. 2022;29(7):1135-53. e8.
39. Kanber
D, Woestefeld J, Döpper H, Bozet M, Brenzel A, Altmüller J, et al. RB1-Negative
Retinal Organoids Display Proliferation of Cone Photoreceptors and Loss of
Retinal Differentiation. Cancers (Basel). 2022;14(9).
40. Pallavi
R, Soni BL, Jha GK, Sanyal S, Fatima A, Kaliki S. Tumor heterogeneity in
retinoblastoma: a literature review. Cancer Metastasis Rev. 2025;44(2):46.
41. Yan
HHN, Chan AS, Lai FP-L, Leung SY. Organoid cultures for cancer modeling. Cell
Stem Cell. 2023;30(7):917-37.
42. Ludwig
AL, Mayerl SJ, Gao Y, Banghart M, Bacig C, Fernandez Zepeda MA, et al.
Re-formation of synaptic connectivity in dissociated human stem cell-derived
retinal organoid cultures. Proc Natl Acad Sci U S A. 2023;120(2):e2213418120.
43. Kandoi
S, Lamba DA. Retinal Organoids: A Human Model System for Development, Diseases,
and Therapies. Adv Exp Med Biol. 2023;1415:549-54.
44. Tao
Y, Hou X, Zuo F, Li X, Pang Y, Jiang G. Application of nanoparticle-based siRNA
and CRISPR/Cas9 delivery systems in gene-targeted therapy. Taylor &
Francis; 2019. p. 511-4.
45. Riba
A, Emmenlauer M, Chen A, Sigoillot F, Cong F, Dehio C, et al. Explicit modeling
of siRNA-dependent on-and off-target repression improves the interpretation of
screening results. Cell systems. 2017;4(2):182-93. e4.
46. Spurgeon
ME, Cheng J, Ward-Shaw E, Dick FA, DeCaprio JA, Lambert PF. Merkel cell
polyomavirus large T antigen binding to pRb promotes skin hyperplasia and tumor
development. PLoS Pathogens. 2022;18(5):e1010551.
47. Zhang
X, Wang W, Jin Z-B. Retinal organoids as models for development and diseases.
Cell Regeneration. 2021;10(1):33.
48. Chehelgerdi
M, Behdarvand Dehkordi F, Chehelgerdi M, Kabiri H, Salehian-Dehkordi H,
Abdolvand M, et al. Exploring the promising potential of induced pluripotent
stem cells in cancer research and therapy. Mol Cancer. 2023;22(1):189.
49. Reynolds
DS, Tevis KM, Blessing WA, Colson YL, Zaman MH, Grinstaff MW. Breast cancer
spheroids reveal a differential cancer stem cell response to chemotherapeutic
treatment. Scientific reports. 2017;7(1):10382.
50. Zhao
Y, Lu T, Song Y, Wen Y, Deng Z, Fan J, et al. Cancer Cells Enter an Adaptive
Persistence to Survive Radiotherapy and Repopulate Tumor. Adv Sci (Weinh).
2023;10(8):e2204177.
51. Takebe
N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, et al. Targeting Notch,
Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin
Oncol. 2015;12(8):445-64.
52. Haddadin
L, Sun X. Stem Cells in Cancer: From Mechanisms to Therapeutic Strategies.
Cells. 2025;14(7).
53. Masoumi
J, Jafarzadeh A, Abdolalizadeh J, Khan H, Philippe J, Mirzaei H, Mirzaei HR.
Cancer stem cell-targeted chimeric antigen receptor (CAR)-T cell therapy:
Challenges and prospects. Acta Pharm Sin B. 2021;11(7):1721-39.
54. Cao
J, Bhatnagar S, Wang J, Qi X, Prabha S, Panyam J. Cancer stem cells and
strategies for targeted drug delivery. Drug Deliv Transl Res.
2021;11(5):1779-805.
55. Kukal
S, Guin D, Rawat C, Bora S, Mishra MK, Sharma P, et al. Multidrug efflux
transporter ABCG2: expression and regulation. Cell Mol Life Sci.
2021;78(21-22):6887-939.
56. Muñoz
P, Iliou MS, Esteller M. Epigenetic alterations involved in cancer stem cell
reprogramming. Mol Oncol. 2012;6(6):620-36.
57. Wu
HJ, Chu PY. Epigenetic Regulation of Breast Cancer Stem Cells Contributing to
Carcinogenesis and Therapeutic Implications. Int J Mol Sci. 2021;22(15).
58. Raval
V, Parulekar M, Singh AD. Emerging New Therapeutics for Retinoblastoma. Ocul
Oncol Pathol. 2022;8(3):149-55.
59. Najafzadeh
B, Asadzadeh Z, Motafakker Azad R, Mokhtarzadeh A, Baghbanzadeh A, Alemohammad
H, et al. The oncogenic potential of NANOG: An important cancer induction
mediator. Journal of cellular physiology. 2021;236(4):2443-58.
60. Takebe
N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, et al. Targeting Notch,
Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nature
reviews Clinical oncology. 2015;12(8):445-64.
61. Amakye
D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the Hedgehog
pathway in cancer. Nature medicine. 2013;19(11):1410-22.
62. Zhang
W, Zhou Q, Wei Y, Da M, Zhang C, Zhong J, et al. The exosome-mediated
PI3k/Akt/mTOR signaling pathway in cervical cancer. International journal of
clinical and experimental pathology. 2019;12(7):2474.
63. Xia
P, Xu XY. PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic
research to clinical application. Am J Cancer Res. 2015;5(5):1602-9.
64. Mohapatra
P, Singh P, Sahoo SK. Phytonanomedicine: A novel avenue to treat recurrent
cancer by targeting cancer stem cells. Drug Discovery Today.
2020;25(8):1307-21.
65. Shibata
M, Hoque MO. Targeting Cancer Stem Cells: A Strategy for Effective Eradication
of Cancer. Cancers (Basel). 2019;11(5).
66. Soliman
H, Theret M, Scott W, Hill L, Underhill TM, Hinz B, Rossi FMV. Multipotent
stromal cells: One name, multiple identities. Cell Stem Cell.
2021;28(10):1690-707.
67. Han
Y, Yang J, Fang J, Zhou Y, Candi E, Wang J, et al. The secretion profile of
mesenchymal stem cells and potential applications in treating human diseases.
Signal Transduct Target Ther. 2022;7(1):92.
68. Chen
L, Liu G, Wu J, Zhou X, Zhao Y, Chen Z, et al. Multi-faceted effects of
mesenchymal stem cells (MSCs) determined by immune microenvironment and their
implications on MSC/biomaterial-based inflammatory disease therapy. Applied
Materials Today. 2020;18:100485.
69. Lou
G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new
therapeutic strategy for liver diseases. Experimental & molecular medicine.
2017;49(6):e346-e.
70. Kalluri
R, LeBleu VS. The biology, function, and biomedical applications of exosomes.
science. 2020;367(6478):eaau6977.
71. Alcayaga-Miranda
F, Varas-Godoy M, Khoury M. Harnessing the angiogenic potential of stem
cell‐derived exosomes for vascular regeneration. Stem cells
international. 2016;2016(1):3409169.
72. Yuan
Ql, Zhang Yg, Chen Q. Mesenchymal stem cell (MSC)‐derived extracellular
vesicles: potential therapeutics as MSC trophic mediators in regenerative
medicine. The Anatomical Record. 2020;303(6):1735-42.
73. Chen
Y, Li J, Ma B, Li N, Wang S, Sun Z, et al. MSC-derived exosomes promote
recovery from traumatic brain injury via microglia/macrophages in rat. Aging
(albany NY). 2020;12(18):18274.
74. Sharma
A, Rani R. Do we really need to differentiate mesenchymal stem cells into
insulin-producing cells for attenuation of the autoimmune responses in type 1
diabetes: immunoprophylactic effects of precursors to insulin-producing cells.
Stem Cell Research & Therapy. 2017;8(1):167.
75. Wang
L, Huang S, Li S, Li M, Shi J, Bai W, et al. Efficacy and Safety of Umbilical
Cord Mesenchymal Stem Cell Therapy for Rheumatoid Arthritis Patients: A
Prospective Phase I/II Study. Drug Des Devel Ther. 2019;13:4331-40.
76. Lotfy
A, AboQuella NM, Wang H. Mesenchymal stromal/stem cell (MSC)-derived exosomes
in clinical trials. Stem Cell Res Ther. 2023;14(1):66.
77. He
X, Dong Z, Cao Y, Wang H, Liu S, Liao L, et al. MSC‐derived exosome
promotes M2 polarization and enhances cutaneous wound healing. Stem cells
international. 2019;2019(1):7132708.
78. Li
D, Yang Y, Zheng G, Meng L, Shang L, Ren J, et al. The potential of cellular
homing behavior in tumor immunotherapy: from basic discoveries to clinical
applications of immune, mesenchymal stem, and cancer cell homing. Front
Immunol. 2024;15:1495978.
79. Yi
BR, Choi KJ, Kim SU, Choi KC. Therapeutic potential of stem cells expressing
suicide genes that selectively target human breast cancer cells: evidence that
they exert tumoricidal effects via tumor tropism (review). Int J Oncol.
2012;41(3):798-804.
80. Choi
KJ, Nam J-K, Kim J-H, Choi S-H, Lee Y-J. Endothelial-to-mesenchymal transition
in anticancer therapy and normal tissue damage. Experimental & molecular
medicine. 2020;52(5):781-92.
81. Wei
J, Han X, Bo J, Han W. Target selection for CAR-T therapy. Journal of
hematology & oncology. 2019;12(1):62.
82. Ma
N, Gao J, Pang X, Wu K, Yang S, Wei H, Hao Y. Formulation-optimized oncolytic
viruses: Advancing systemic delivery and immune amplification. J Control
Release. 2025;383:113822.
83. Helmink
BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary
lymphoid structures promote immunotherapy response. Nature.
2020;577(7791):549-55.
84. Yuan
M, Huang L-L, Chen J-H, Wu J, Xu Q. The emerging treatment landscape of
targeted therapy in non-small-cell lung cancer. Signal transduction and
targeted therapy. 2019;4(1):61.
85. Klopp
P, Griebner U, Zorn M, Weyers M. Pulse repetition rate up to 92 GHz or pulse
duration shorter than 110 fs from a mode-locked semiconductor disk laser.
Applied Physics Letters. 2011;98(7).
86. Fu
T, Ma Y, Jiang Y, Jiang C, Li X, Jiang Q. Role of stem cells in ocular
diseases: Progress and challenges. J Biomed Res. 2025:1-21.
87. Sadiq
IZ, Abubakar FS, Katsayal BS, Ibrahim B, Adamu A, Usman MA, et al. Stem cells
in regenerative medicine: Unlocking therapeutic potential through stem cell
therapy, 3D bioprinting, gene editing, and drug discovery. Biomedical
Engineering Advances. 2025;9:100172.
88. Wang
Y, Fang J, Liu B, Shao C, Shi Y. Reciprocal regulation of mesenchymal stem
cells and immune responses. Cell stem cell. 2022;29(11):1515-30.
89. Al-Ansi
A, Han H. Role of halal-friendly destination performances, value, satisfaction,
and trust in generating destination image and loyalty. Journal of destination
marketing & management. 2019;13:51-60.